ICP-MS Configuration and Optimization for Successful Routine Analysis of Undiluted Seawater
Inductively coupled plasma–mass spectrometry (ICP-MS) is a technique increasingly being used in commercial laboratories and industries that analyze samples with complex matrices, such as seawater, wastewater, saline groundwater, and other high salt samples. These laboratories often choose ICP-MS for its fast, multielement capability and high sample throughput, but time and cost pressures mean that laboratories have little time for sample preparation and method development. The time and cost pressures can lead to performance and operational issues because an ICP-MS instrument optimized for the highest sensitivity may not have the sufficient matrix tolerance to analyze high salt samples. In this article, we describe a method to optimize plasma robustness and interference control that enables a modern ICP-MS to perform simple, accurate, and routine analysis of critical trace elements in undiluted seawater.
As inductively coupled plasma mass spectrometry (ICP-MS) instruments become more widely available, less expensive, and easier to use, the technique is increasingly being used for routine trace element analysis in more challenging samples. High and variable matrix levels can be a feature of samples from many different industries and applications. Examples include food and agriculture, pharmaceuticals, chemical and petrochemical manufacturing, mineral prospecting, clinical research, and environmental analysis of groundwater, wastewater, and seawater.
Trace element levels in coastal and estuarine water are monitored to ensure the quality and safety of waters used for fishing and shellfish farming, as well as for recreational activities. Coastal seawaters contain higher concentrations of trace elements than open-ocean seawater, but priority contaminants such as toxic heavy metals must still be measured at low and sub-ppb (μg/L) concentrations, which means ICP-MS is often the preferred technique. Sample salinity in estuaries and coastal regions varies because of freshwater mixing, giving a range from seawater at approximately 3.5% total dissolved solids (TDS) to low salinity river water. As a result, routine ICP-MS methods must provide accurate analysis for varied sample matrices, a challenge that is further complicated by the fact that estuarine and coastal waters can contain high levels of dissolved minerals and organic matter.
Traditional ICP-MS methods for analyzing saline waters typically involve diluting the samples to reduce the salt matrix to below 0.2%, or 2000 ppm, TDS. This historical TDS limit has long been accepted as a requirement for routine ICP-MS analysis and has also been adopted in several standard ICP-MS methods, such as EN-ISO 17294-2 (Water Quality), US-EPA 6020B (Water and Wastes), and EN 13805 (Trace Elements in Food). However, diluting samples—either manually or using an autodilutor—degrades detection limits and risks adding contamination from the diluent and the laboratory equipment used to process the samples. The relative contribution from contaminants in the diluent rises as the dilution factor increases, so laboratories must take care to avoid errors being multiplied in the dilution-corrected concentrations reported in the original samples. Therefore, routine laboratories often prefer simple and more consistent methods where samples are analyzed directly without dilution.
Minimizing or avoiding sample dilution helps reduce contamination but results in higher matrix levels, potentially far above the accepted 0.2% TDS limit. Some modern ICP-MS systems have been engineered to tolerate high salt matrices for extended periods, offering an alternative to liquid dilution. One approach, known as aerosol dilution, dilutes the samples in the aerosol state, using argon gas as the diluent (1). Aerosol dilution avoids time-consuming sample handling steps and eliminates contamination from liquid diluents. Another benefit of aerosol dilution is that it reduces the aerosol load reaching the plasma, which results in a higher effective plasma temperature. High matrix levels require more plasma energy to decompose, so the plasma must be optimized for maximum robustness (lowest CeO+/Ce+ ratio) to ensure it has sufficient energy to fully decompose the matrix. Commercial ICP-MS instruments range from a CeO/Ce ratio of approximately 0.03 (3%) for the least robust plasma to approximately 0.01 (1%) for the most robust plasma. Using aerosol dilution further improves the plasma robustness to a CeO/Ce ratio of approximately <0.005 (0.5%). Under these very robust, low CeO/Ce plasma conditions, undiluted seawater can be measured for extended periods without significant signal drift because of matrix deposition on the interface cones.
A further issue that can affect ICP-MS analysis of high salt samples is loss of analyte sensitivity because of ionization suppression. This effect occurs when free electrons from easily ionized major elements—such as sodium, potassium, and calcium—suppress the ionization of less easily ionized analyte elements, leading to lower analyte sensitivity. Ionization suppression affects poorly ionized elements to a greater degree and unfortunately several critical analytes—including arsenic, cadmium, and mercury—are poorly ionized. Using more robust plasma conditions reduces ionization suppression, increasing the signal for poorly ionized analytes and giving a more consistent analyte signal in varying levels of easily ionized matrix elements (2).
Finally, the high levels of several major elements in seawater can cause matrix-based polyatomic ion overlaps that lead to errors in the results. Avoiding, correcting, or resolving such spectral overlaps is a key part of developing a robust and reliable method for saline water samples. Some of the matrix-based polyatomic ion overlaps that can affect common analytes in saline samples, such as seawater, are shown in Table I. Mathematical interelement correction equations have historically been used in ICP-MS methods to correct the contribution from certain polyatomic ions, and such equations are still defined in some standard methods, such as EPA 200.8. However, these corrections are not always reliable in complex samples. For example, a standard correction equation for arsenic corrects for the argon chloride overlap on arsenic at mass 75. Argon chloride-75 is calculated from argon chloride-77 after subtracting the contribution from the selenium-77 signal, which is in turn calculated from the selenium-82 signal after correction for the contribution from krypton-82. However, this correction fails if significant bromine or sulfur is present in the sample because mass 82 also suffers overlaps from BrH+, So3+, and S2O+.
Most modern ICP-MS instruments now include a collision–reaction cell (CRC), which can give much more effective and reliable control of the polyatomic ions. CRCs use either reaction chemistry or collision mode, using a reactive or an inert cell gas, respectively. Reactive cell gases can be used on single quadrupole ICP-MS, but they have limited applicability as reactive gases can lead to significant new errors because of cell-formed reaction product ions. Consequently, on single quadrupole ICP-MS, collision mode using helium (He) cell gas is a much more reliable and universal approach, which is especially true for multielement analysis in high matrix samples because the inert He cell gas eliminates the possibility of new reaction product ions being formed from matrix elements. With an optimized cell configuration and operating conditions, polyatomic ions can be filtered out effectively using helium cell gas and kinetic energy discrimination (KED). KED works for all polyatomic ions, so the same cell conditions can be applied successfully to multiple interferences, multiple analytes, and for complex and varied sample types (3).
Experimental
A standard Agilent 7900 ICP-MS instrument fitted with the ultrahigh matrix introduction (UHMI) aerosol dilution system was used for all measurements. UHMI utilizes the standard peristaltic pump and concentric nebulizer, but the plasma is optimized for highly robust conditions, with a longer sampling depth, a reduced carrier gas flow rate, and an additional dilution gas flow. A standard glass concentric nebulizer, a quartz-spray chamber temperature controlled to 2 °C, and a quartz torch with a 2.5-mm injector, were used together with standard nickel sampling and skimmer cones. Analyte isotopes, integration times, and cell conditions were defined by the selected preset method (EPA 6020). Plasma conditions were defined by the UHMI setting, and other instrument tune settings were optimized automatically using the ICP-MS MassHunter autotune function. No manual optimization was required.
The Agilent 7900 was used to analyze multiple elements of interest in two natural seawater samples known to contain low levels of trace elements. Samples were measured with and without a 10 μg/L multielement spike, with the results being calibrated against simple (no salt matrix), acidified, aqueous calibration standards. The standards were prepared from a mixed stock standard (multielement calibration standard 2A, Agilent part number 8500-6948) and single element standards for molybdenum, antimony, tin, thorium, and mercury (Kanto Chemical Co). The standards were diluted using ultrapure, 18 MΩ cm water (Millipore) and acidified to 1% nitric acid (HNO3) and 0.5% hydrogen chloride (HCl) (Kanto Kagaku EL grade).
Seawater contains approximately 3.5% total dissolved solids, but the matrix is mostly sodium chloride (NaCl), which is relatively easily dissociated in the ICP plasma. Therefore, undiluted seawater can be run on an Agilent 7900 ICP-MS instrument using an aerosol dilution setting of UHMI-8 (approximately 8× aerosol dilution). As is normal when analyzing high salt matrices, an argon humidifier accessory was used to reduce the buildup of salt crystals on the nebulizer and torch injector. Normal internal standard (ISTD) pump tubing with an internal diameter (i.d.) of 0.25 mm was used for the online addition of the ISTD solution. The sample solution was pumped using the standard 1.02-mm i.d. tubing, so the sample matrix was only diluted approximately 6% by the addition of the ISTD solution prior to nebulization.
The Agilent 7900 is typically optimized for robust plasma conditions, indicated by a low CeO/Ce ratio of approximately 0.012 (1.2%). UHMI conditions further improve plasma robustness to a CeO/Ce ratio of approximately 0.005 (0.5%), which ensures effective ionization of high first ionization potential (IP) elements, such as beryllium, zinc, arsenic, selenium, cadmium, and mercury, even in the presence of a high level of easily ionized matrix elements. No special sample preparation or operating conditions—such as the addition of carbon to the sample solutions and N2 gas to the plasma—were required.
The EPA 6020 preset method used includes both no gas and He mode tune steps, but only the helium mode results are reported in this work. With the octopole reaction system (ORS) cell used in the Agilent 7900, helium mode conditions maintained high ion transmission while providing the high cell gas pressure required for effective interference removal by KED. Operating conditions used for the analysis of undiluted seawater are shown in Table II.
Results and Discussion
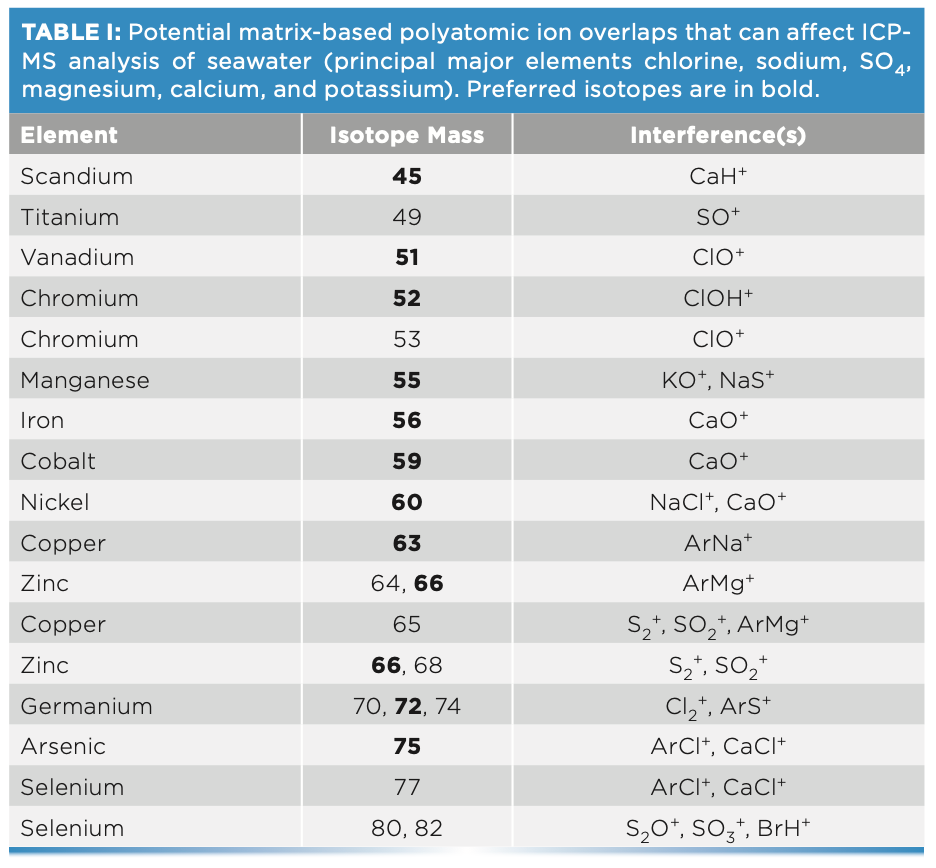
To validate the plasma conditions used for undiluted seawater analysis, samples containing a range of salinity levels were measured to assess the ionization suppression for the poorly ionized element cadmium. The saline samples were measured under several different plasma conditions covering the range typically reported for commercial ICP-MS systems, from the least to the most robust plasma conditions (CeO/Ce ratios from 0.03 (3%) to 0.005 (0.5%) with aerosol dilution). Cadmium, which has a high first ionization potential (1st IP) of 8.994 eV, was measured in various concentrations of salt matrix from 0.035% TDS (100× diluted seawater) to 3.5% TDS (undiluted seawater). Cadmium recovery was calculated for each salt matrix, calibrated using simple calibration standards (no salt matrix). The recoveries, shown in Figure 1, indicate that robust plasma conditions (CeO/Ce of 0.5%) gave consistent and accurate results for cadmium, eliminating the suppression observed under less robust (higher CeO/Ce) conditions. Robust conditions increase the amount of plasma energy available, giving higher and more consistent ionization of poorly ionized elements such as cadmium.
FIGURE 1: More robust plasma (lower CeO/Ce ratio) reduces ionization suppression, improving the recovery of cadmium (1st ionization potential 8.994 eV) in the presence of variable salt concentrations up to ~3.5% (undiluted seawater).
Spike Recovery and Stability in Seawater Reference Materials
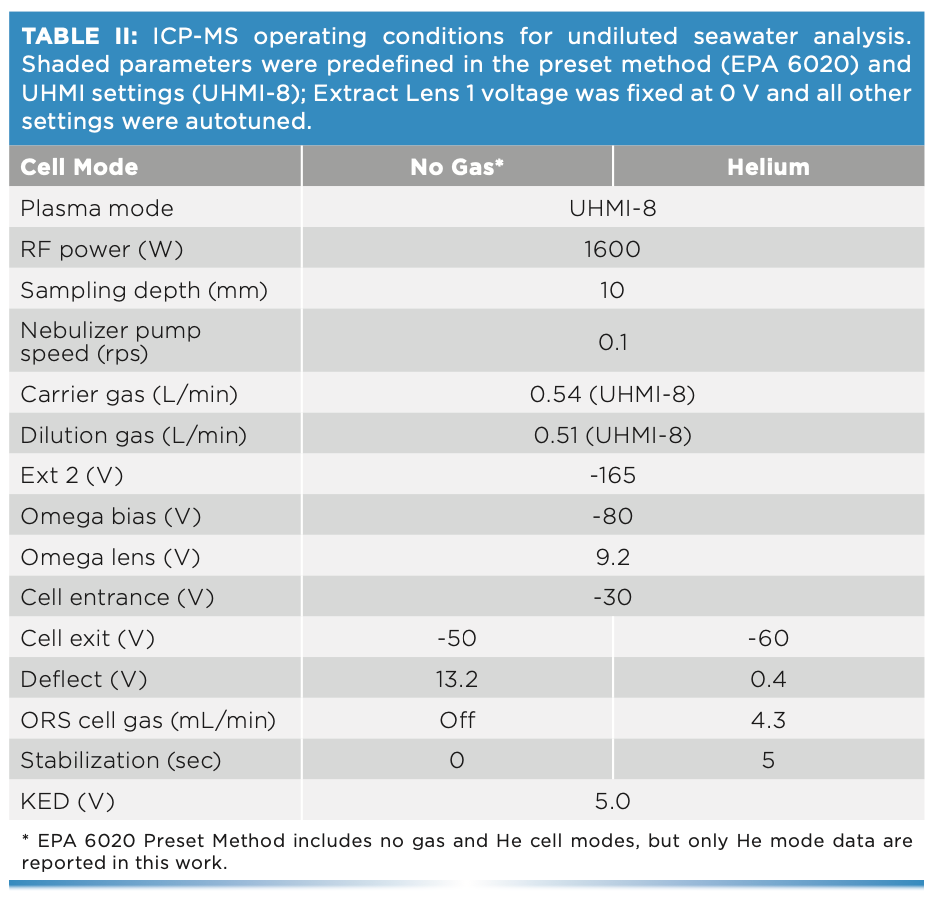
Using the UHMI-8 conditions (CeO/ Ce ratio of ~0.005 (0.5%)), the two different undiluted seawater samples were analyzed alternately, with and without a spike containing the analytes at 10 μg/L (mercury at 1 μg/L), over a period of 9 1⁄2 h. Trace element concentrations were quantified against simple aqueous calibration standards. The measured concentrations of one analyte, manganese, in the alternating spiked and unspiked samples are plotted in Figure 2. The mean spike recoveries for all elements are shown in Figure 3. Excellent recoveries of the spike concentrations were achieved, with most elements within +/- 5% of the spiked value, and with precision better than 5% RSD for the entire 9 1⁄2-h sequence.
FIGURE 2: Long-term results for manganese in alternating unspiked and spiked (10 μg/L) undiluted seawater samples measured over 9 1⁄2 h.
FIGURE 3: Spike recoveries of 10 μg/L spike in undiluted seawater samples analyzed over 9 1⁄2 h. Robust plasma conditions and helium cell mode gave good control of ionization suppression and matrix-based polyatomic ions, allowing all elements to be measured at their preferred isotopes, including V-51, Cr-52, Cu-63, As-75, and Se-78. Seawater A is the blue bar, and seawater B is the orange bar.
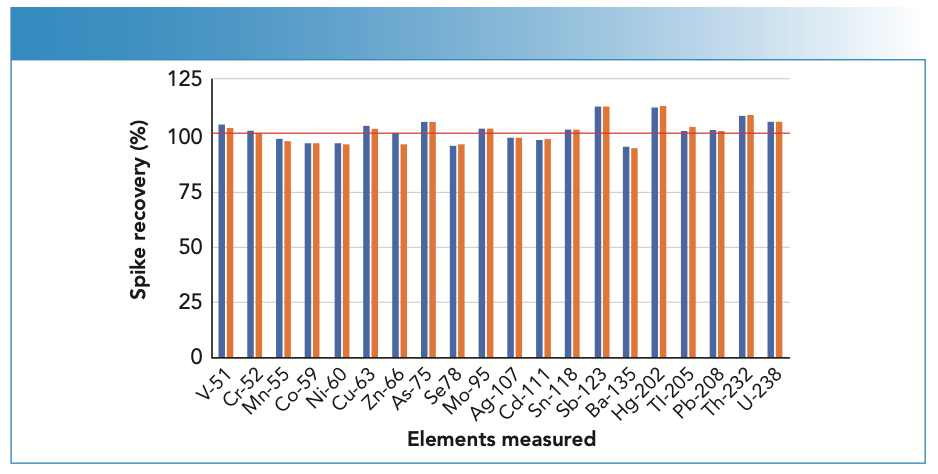
The good recovery of the poorly ionized elements zinc, arsenic, selenium, cadmium, and mercury in the undiluted seawater matrix—measured against non-matrix-matched calibration standards—demonstrates the good control of ionization suppression. This is particularly important for applications where sample composition varies between samples, such as in the analysis of mixed saline environmental waters, where salt levels vary dramatically between river, estuarine, and seawater. Good recoveries for the interfered elements confirm the effective control of polyatomic ion overlaps using helium KED.
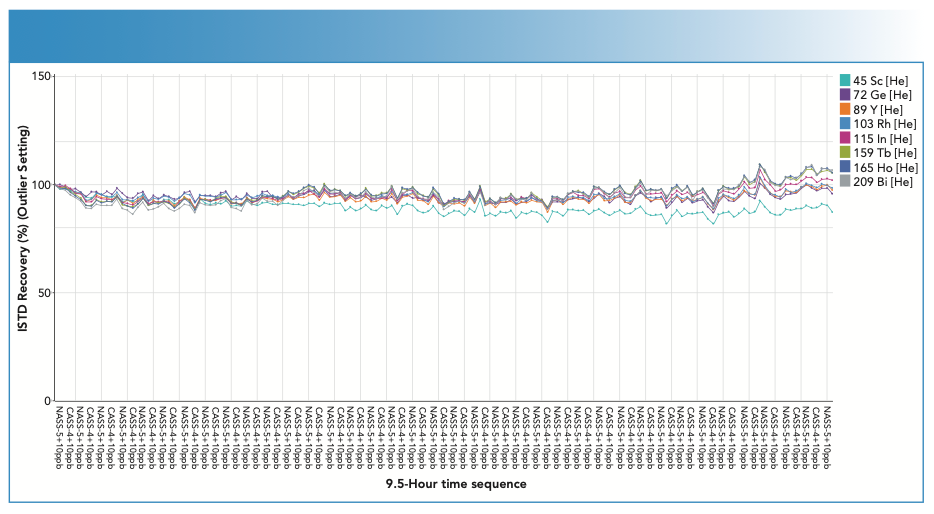
For any ICP-MS application that involves analysis of high matrix samples, it is recommended that ISTD signals are monitored throughout the analytical sequence. The ISTDs correct for the physical differences in sample transport and nebulization between low matrix (for example, simple calibration standards) and high matrix (such as undiluted seawater) solutions. The ISTD stability plot for each batch also provides an indication of the consistency of the raw signals measured by the ICP-MS, which is useful for monitoring drift and suppression. Figure 4 shows the ISTD signals from the long-term analysis of undiluted seawater samples. The robust UHMI conditions used ensured that there was minimal matrix deposition and signal drift throughout the 9 1⁄2-h analysis.
FIGURE 4: Internal standard (ISTD) signals showed stable long-term analysis of unspiked and spiked undiluted seawater samples analyzed during a 9 1⁄2-h sequence. ISTD signals remained within ±20% QC limits, indicated by the red dotted lines. Because of limited space, not all sample names are displayed.
Conclusions
The Agilent 7900 ICP-MS instrument with UHMI aerosol dilution can tolerate high sample matrix levels, allowing stable, accurate, long-term analysis of samples such as seawater without needing either online dilution or manual sample dilution prior to analysis. UHMI aerosol dilution provides fully automated and calibrated setup of plasma conditions and aerosol dilution factors, delivering consistent analytical performance for routine laboratories.
Very robust plasma conditions (CeO/Ce ratio of 0.005 or 0.5%) reduce ionization suppression, increase sensitivity, and improve detection limits for critical poorly ionized elements such as arsenic, cadmium, and mercury. Reduced ionization suppression allows high and variable sample matrices to be measured against simple aqueous calibration standards, eliminating the need for matrix matching of calibration standards. This provides a greatly simplified workflow in laboratories that analyze samples with unknown or variable composition.
In this study, an Agilent 7900 ICP-MS with UHMI aerosol dilution was used to analyze a range of trace elements in undiluted seawater samples. The accurate spike recoveries against simple aqueous standards and the excellent long-term stability demonstrate the matrix tolerance and simple operation offered by the method.
References
(1) S.M. Wilbur and L.C. Jones, The Open Chemical and Biomedical Methods Journal 3, 135–142 (2010).
(2) E. McCurdy and W. Proper, Spectroscopy s11(29), 40–47 (2014).
(3) T. Kubota, “Analysis of Undiluted Seawater using ICP-MS with Ultra High Matrix Introduction and Discrete Sampling,” Agilent Publication 5994-4467 EN (2021).
Tetsushi Sakai and Ed McCurdy are with Agilent Technologies, Inc. Direct correspondence to: ed_mccurdy@agilent.com ●
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
