Initial Quantitative Measurements with Handheld LIBS
Spectroscopy
The analytical technique laser induced breakdown spectroscopy (LIBS) for elemental analysis is rapidly evolving from a laboratory-based to a field-able technology. Keys to this evolution include the development of more powerful yet compact, battery operable lasers, and combining the advancing laser technology with powerful processors and compact, high resolution spectrometers. The result is the first generation of truly handheld LIBS-based analyzers. One such analyzer, the SciAps Z-500 LIBZ analyzer, has been utilized in a number of studies of different material types including alloys, ceramics, catalysts, soils, and glass sample types. This note presents the early quantitative results from several field tests.
The Z-500 utilizes a 5 mJ, 10 Hz, pulsed laser that operates at an eye-safe wavelength of 1532 nm. It possesses a proprietary spectrometer that spans a wavelength range of 185–680 nm, with 0.1 nm (FWHM) resolution. The laser is focused down to approximately a 50 µm spot to produce a bright plasma at the sample surface. The focusing lens and collection optics are mounted on a 3D stage under processor control. This allows the laser to be rastered around the sample, to collect spectra from multiple locations, which the analyzer averages to account for sample non-homogeneity, and for automated focal length adjustments. The spectrometer is gated to reject the large amount of background radiation emitted in the early stage of the plasma, largely due to Bremsstrahlung radiation. The region around the plasma is (optionally) argon-purged with an on-board argon canister stored in the analyzer handle. The argon purge, combined with spectrometer gating improves the limits of detection by a factor of 5×–20× (element dependent) due to both increased signal from the inert gas environment, and greatly reduced background from the spectrometer gating.
Application Examples
The first example is the analysis of ceramic samples. Ceramics are highly heat resistant and were anticipated to be a challenge to ablate with a 5 mJ laser. However, as shown in Figure 1 for several commercially available ceramics, the response for Mg concentration is reasonably good. Mg was the only element of interest for this particular study. In this study, several ceramic samples of known Mg content were tested in three locations and the spectra were averaged. Thus, each data point in Figure 1 is an average of 60 laser pulses, at three distinct locations on the sample, for a total of 180 pulses. The test time was 18 s with the laser operating at 10 Hz. The Mg intensity was divided by a sum of multiple elements in the spectrum to produce the intensity rations shown.
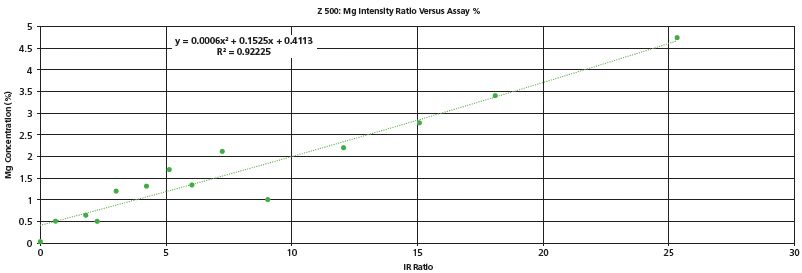
Figure 1: Analysis of ceramic samples for Mg content.
Catalysts
Several car catalyst samples were analyzed. Elements of interest include Pt, Pd, Rh, and Ce. Results from a set of four samples are shown in Figure 2 a–d. The catalyst samples were lightly pressed into sample cups. They were analyzed in 20 different raster spots over a 6 s test time. Three sub-samples were analyzed for a total test time of 18 s. Detection limits from this first pass data are estimated as < 30 ppm (Rh), < 100 ppm (Pd), < 100 ppm (Ce), and < 300 ppm (Pt). We anticipate that limits of detection will improve as a field-able pressing method is developed.
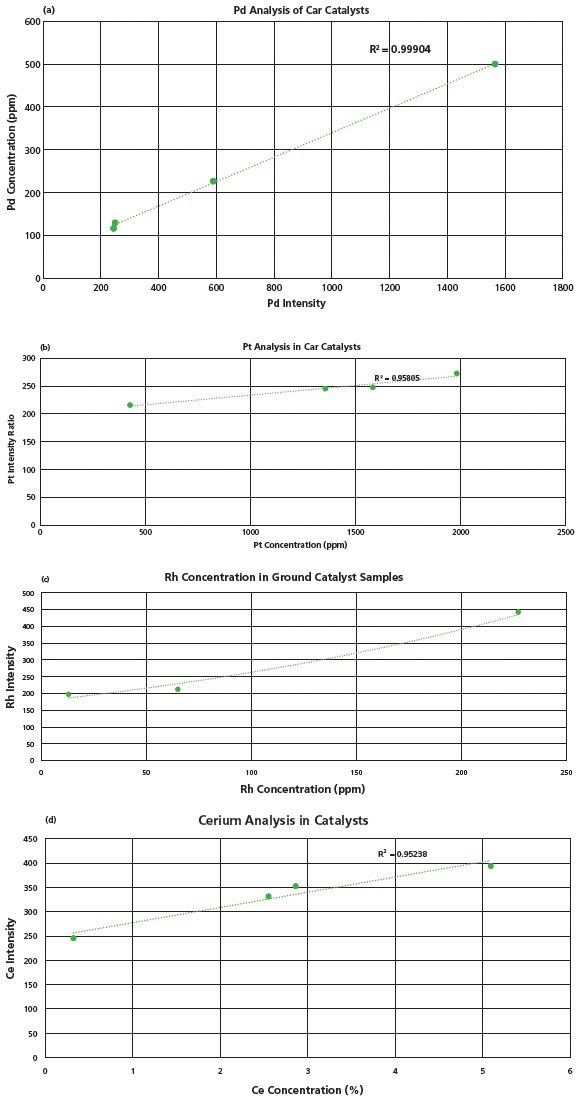
Figure 2: (a–d): Results from catalyst analysis for Pd, Pt, Rh, and Ce. Rh exhibited the best limit of detection at < 30 pppm.
The detection of Ce is interesting because it's a rare earth element (REE). REE's are widely sought after, yet difficult to measure with field portable XRF because the main X-ray excitations are nested within the emissions of several common transition metals, thus elevating real-world limits of detection. Handheld LIBS may offer an alternative technique that is more sensitive, without the need for higher powered X-ray sources.
Be in Soil
Beryllium in soil was also analyzed by the Z-500. Beryllium is often of interest as an environmental contaminant or as an identifier of specific minerals of commercial interest. It's one of 13 EPA Priority Pollutant metals, but cannot be analyzed by field portable XRF because the emitted X-rays are too low in energy. Shown in Figures 3a and 3b are results and spectra from Be samples of 131 ppm (red) and 500 ppm (green). The results from a field blank are also shown. These data were collected with a test time of 4 s. The two points shown at each sample in Figure 3a represent the ranges of the Be intensity ratio from repeat measurements.The limit of detection is estimated at 10 ppm, and the three samples, including the blank, were very linear in response. The samples were reasonably homogenized to approximately a 200 µm particle size or less, and were each about 50 g of soil. They were analyzed repeatedly in the same region, and also analyzed at three different regions within the soil bags. The spectra shown are averages of the repeats, at each of the three different locations of the main sample. The variability between the three spectra at each concentration are largely due to sample non-homogeneity, rather than instrument repeatability.
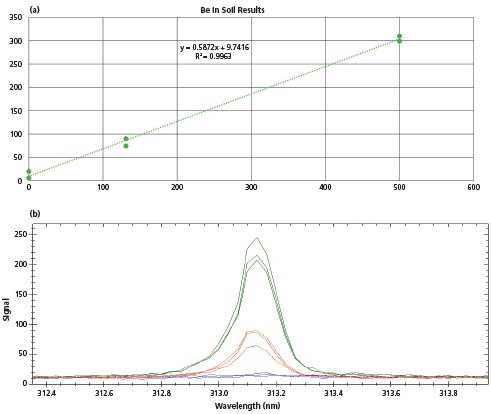
Figure 3: (a–b) Results and spectra for Be in soil from a blank
Nickel Content in Stainless Steel
A final example of the quantification ability for handheld LIBS is the analysis of nickel in stainless steels. Ni analysis is important both for quality control applications for alloys (Positive Materials Identification) and for scrap metal sorting. Therefore, accurate nickel measurements are a key requirement in the alloy identification field. Figure 4 shows the response curve to a wide range of nickel concentrations in stainless steels. These range from a low of 0.2% in a 410 stainless up to 25% Ni in A286. The data shown were for 6 s tests on a set of 20 certified reference alloys for stainless steel. Plotted are the nickel intensity ratio's (referenced to Fe) versus Ni assay. The Ni correlation is very good over two orders of magnitude in nickel concentration.
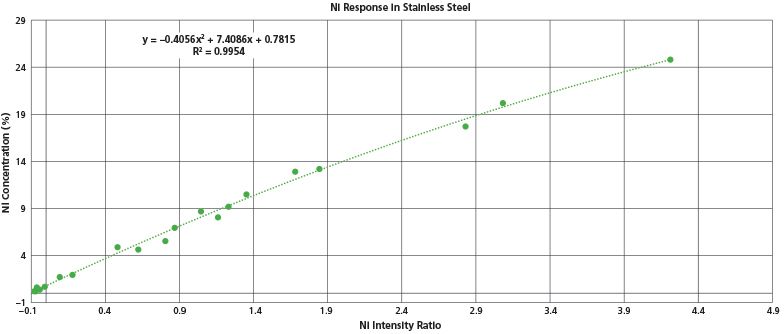
Figure 4: Calibration curve for nickel content in stainless steels, ranging from 0.2% up to 25%.
In summary, a novel handheld LIBS spectrometer has been developed to identify and quantify elemental concentrations in a variety of sample types. Initial data has been collected for representative sample types: soils, ores, catalyst material, and alloys. Data has also been collected for specific elements in glass and ceramics with encouraging results (not shown due to space considerations). Testing times are typically 6 s or less, or at most 18 s if up to three tests are averaged together. The data show that handheld LIBS is a viable field analytical technique. A new generation of lasers that are higher powered and with further improvement in beam quality are expected to contribute the most to further improvements in analytical performance.

SciAps, Inc.
2 Constitution Way, Woburn, MA 01801
tel. (339) 927-9455
Website: www.sciaps.com

Atomic Perspectives: Highlights from Recent Columns
March 3rd 2025“Atomic Perspectives,” provides tutorials and updates on new analytical atomic spectroscopy techniques in a broad range of applications, including environmental analysis, food and beverage analysis, and space exploration, to name a few. Here, we present a compilation of some of the most popular columns.
Pittcon 2025: Highlighting Talks on Atomic Spectroscopy
February 26th 2025At Pittcon this year, there will be numerous sessions dedicated to spotlighting the latest research that uses atomic spectroscopy or elemental analysis techniques. We highlight some of these talks below that might pique the interest of spectroscopists and researchers attending the conference this year.