Key Steps in Method Development for Inductively Coupled Plasma–Tandem Mass Spectrometry (ICP-MS/MS)
Inductively coupled plasma–tandem mass spectrometry (ICP-MS/MS) is a relatively new technique, with the first triple quadrupole ICP-MS (ICP-QQQ) being introduced commercially in 2012. However, the technique has quickly been accepted for a wide variety of applications in industries including, but not limited to, manufacturing, contract analysis, government, research, and academia. In this article, we explain the key steps to setting up an ICP-MS/MS method and address some of the common misconceptions surrounding the technique.
Compared to conventional single quadrupole inductively coupled plasma–mass spectrometry (ICP-MS), the main benefits of ICP-MS/MS include:
- the unprecedented ability to resolve spectral interferences using controlled reaction chemistry in the collision reaction cell (CRC);
- significantly better separation of adjacent mass, “peak tail” overlaps because of two mass filtering steps; and
- improved detection limits through higher sensitivity and lower backgrounds.
The flexibility of ICP-MS/MS has led some people to assume that method development must be more difficult on ICP-MS/MS than on single quadrupole ICP-MS. However, existing single quadrupole methods can usually be transferred to ICP-MS/MS without significant modifications. Also, reaction gas methods run more consistently on ICP-MS/MS, so methods are more universal and operation is simpler.
The following six steps help guide method setup for ICP-MS/MS (Figure 1):
FIGURE 1: Method development steps for ICP-MS/MS.
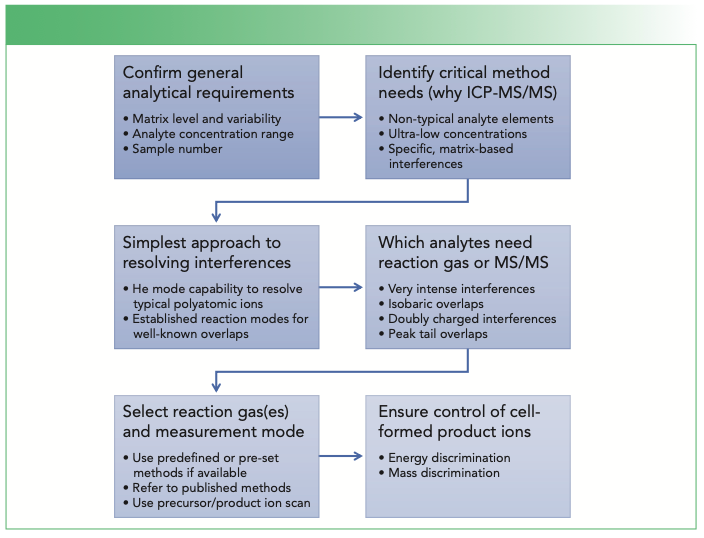
Step #1: Get the Basics Right
When developing an ICP-MS/MS method, users may focus on a specific interference that needs to be resolved. However, the starting point for method development on ICP-MS/MS—as with conventional ICP-MS—should consider the wider analytical needs. These needs include several factors that can be easily overlooked.
First, can the instrument handle the sample matrix? Matrix tolerance is important for many ICP-MS applications. Optimizing the plasma to give low CeO+/ Ce+ (<1.5%) ensures that the instrument will easily tolerate typical sample matrix levels. Low CeO/Ce indicates better matrix decomposition, which reduces deposits on the interface and leads to better stability and less maintenance. Low CeO/Ce conditions also increase analyte ionization, improving sensitivity for poorly ionized elements, such as beryllium, arsenic, cadmium, and mercury. Low CeO/Ce indicates better decomposition of molecular species that would otherwise form polyatomic ion overlaps (1).
Second, is the sample composition unknown or variable? The major element levels and sample-to-sample variability can be important factors. Variable sample matrices can give rise to unexpected polyatomic ion overlaps, which a given reaction gas mode may not resolve effectively. In such cases, helium (He) collision mode may provide a simpler, more universal, and more reliable solution (2,3).
Third, which analytes must be measured and at what level? Most ICP-MS applications can be run successfully using He collision mode, but lower analyte levels may require a reaction gas method to further reduce backgrounds and improve detection limits. Similarly, analysis of high-level major elements may require custom tuning or sample dilution if the detector cannot measure the full concentration range.
Finally, how many samples will be analyzed? Many parameters affect analysis speed for large sample batches. ICP-MS/MS systems are compatible with discrete sampling devices to shorten sample analysis times. However, the number of cell gases used—and the time required to switch between cell modes—will also impact throughput.
Step #2: Identify Critical Method Needs—Why is ICP-MS/MS Needed?
Once the basic analytical requirements have been addressed, specific, critical aspects of the analysis should be considered. This stage usually focuses on the most difficult sample types, unusual analytes, or extremely low (or high) concentration levels. In the context of ICP-MS/MS method development, this step should identify analytes that are affected by spectral overlaps.
Step #3: Apply the Simplest Approach to Address Interferences
ICP-MS/MS offers performance benefits because of the additional quadrupole, Q1, before the CRC, which controls reaction processes in the cell. Reaction gas modes can give extremely effective interference reduction, useful for ultralow level analysis in high purity reagents. However, reaction gas methods may be specific for each overlap being resolved, so multielement methods may require several sets of cell conditions. These conditions may not always be applicable to other sample types.
As mentioned above, many ICP-MS interferences can be addressed using He collision mode. Most spectral overlaps in ICP-MS are caused by polyatomic (molecular) ions. For example, 40Ar35Cl+ overlaps 75As+ at m/z 75. Helium mode uses kinetic energy discrimination (KED) to reduce the transmission of polyatomic ions relative to the monatomic analyte ions they overlap (4). As a result, He mode supports multi-element analysis and allows access to secondary (confirmatory) isotopes for many elements, while also practically eliminating the need for unreliable mathematical correction equations.
The major appeal of He mode is that the same cell conditions can be used for multiple analytes across a range of sample types, so method setup is simple and consistent. He mode is the default mode for single quadrupole ICP-MS methods, and can be used in the same way on ICP-MS/MS. Using He mode for most analytes makes it much simpler to develop methods for the remaining analytes that need a reaction gas approach.
Step #4: Identify Analytes or Overlaps that Cannot Be Addressed Using He Mode
Many users employ ICP-MS/MS precisely because their application cannot be performed successfully using single quadrupole ICP-MS, even with well optimized He mode. Usually, the requirements relate to specific spectral interferences or adjacent mass overlaps that can only be resolved using a reaction gas or double mass selection (MS/MS).
ICP-MS/MS can use reaction gas modes to reduce intense spectral overlaps, such as 14N2 on 28Si, 14N16O1H on 31P, and 16O2 on 32S, allowing these previously difficult elements to be measured at a low level. Reaction gas modes can also reduce matrix-based overlaps to lower levels, enabling ultratrace level analysis.
He mode cannot resolve isobaric overlaps, such as 48Ca on 48Ti, 40Ar on 40Ca, 204Hg on 204Pb, and so on. In typical, routine ICP-MS analysis, these overlaps are avoided by measuring an alternative mass (47Ti, 44Ca, and 206/207/208Pb, in the examples above). However, measurement of the overlapped isotope is required for some applications and provides performance benefits, such as higher sensitivity, in others. In these cases, a reaction gas mode may be able to resolve the overlap.
Doubly charged ion interferences form when an atom loses two electrons in the plasma and forms a doubly charged (M2+) ion. A quadrupole mass filter separates ions based on mass to charge ratio (m/z), so M2+ ions appear at half their true mass. Most elements form insignificant levels of M2+ ions, but some, such as the rare earth elements (REEs), do form a small percentage of M2+ ions. These REE2+ ions can cause overlaps. For example, 150Nd2+ and 150Sm2+ overlap 75As+. Single quadrupole ICP-MS can correct for normal levels of M2+ overlap, but this approach is not effective at high levels of interference. For analyses such as trace arsenic and selenium in REE minerals, a reaction gas method is more effective.
Peak tail overlaps affect trace analytes measured next to intense major element peaks. An example is trace manganese (m/z 55) analysis in an iron or steel matrix (the major isotopes of iron are at m/z 54 and 56). Peak tail overlaps are resolved using the double mass selection of MS/MS, as the abundance sensitivity (AS) of a tandem MS is the product of the AS of the two mass filters, so Q1 AS x Q2 AS in the case of ICP-QQQ.
Step #5: Select the Preferred Reaction Gas Mode
For overlaps that cannot be addressed using He mode, an appropriate reaction gas mode is selected, which is not as daunting as new users often assume, because ICP-MS/MS ion–molecule reaction chemistry is well understood and documented. Applications can often be run using the predefined method settings provided with the instrument software. Manufacturers’ application notes (5) and users’ publications (6) document proven methods for many more applications.
ICP-MS/MS users also benefit from unique method development tools, including product ion and precursor ion scans. These are useful for identifying suitable analyte product ions and potential matrix-based product ion overlaps (7).
Step #6: Ensure Control of Cell-Formed Reaction Product Ions
When reaction gases are used in ICP-MS, new product ions can be formed in the CRC. The creation of potentially interfering product ions is a major downside of reaction gases compared to helium KED mode, but control of product ion formation is a key benefit of a tandem MS configuration. The Q1 mass filter, before the CRC, ensures that only ions at the target mass—that is, the analyte and any on-mass interferences—enter the CRC.
Q1 therefore prevents the formation of product ions from analytes or matrix elements present at other masses. However, product ions can still be created from the reaction gas itself. For example, when 52Cr+ is measured in organic matrices, Q1 is set to m/z 52, allowing both 52Cr+ and 40Ar12C+, to enter the cell. NH3 cell gas removes the 40Ar12C+ via a charge transfer reaction:
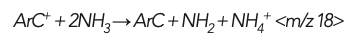
However, the NH4+ product ions could react further with the NH3 cell gas, forming new NH4(NH3)2+ cluster ions at m/z 52:
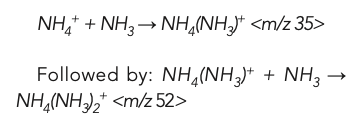
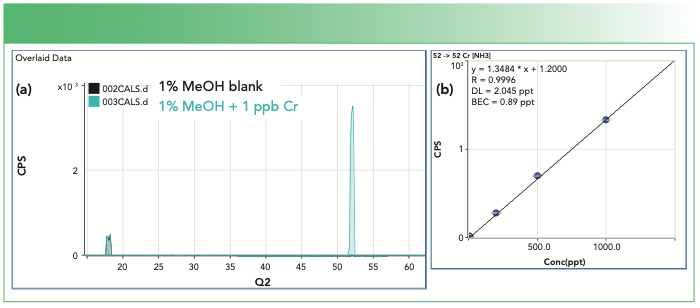
The sequential reactions that form the NH4(NH3)2+ product ions can be prevented using either mass discrimination or ion energy discrimination, depending on the ICP-MS/MS cell configuration and operating conditions. The energy discrimination approach is effective because product ions created from the cell gas only have the kinetic energy they derive from the collision. These low-energy product ions have insufficient energy to pass through the gas-filled cell, so they do not appear in the mass spectrum (Figure 2).
FIGURE 2: (a) Overlaid ICP-MS/MS spectra (blank and 1 ppb chromium (Cr) in 1% methanol). (b) Calibration plot for 52Cr. Background <1 ppt confirms cell-formed NH4(NH3)2+ product ions (m/z 52) are removed using energy discrimination.
References
(1) E. McCurdy and D. Potter, Spectroscopy Europe 13(3), 14,16–20 (2001).
(2) N. Yamada, J. Takahashi, and K. Sakata, J. Anal. At. Spectrom. 17, 1213–1222 (2002).
(3) E. McCurdy and G. Woods, J. Anal. At. Spectrom. 19, 607–615 (2004).
(4) E. McCurdy, N. Sugiyama, and S.M. Wilbur, Spectroscopy 25(11s), 20–29 (2010).
(5) “Handbook of ICP-QQQ Applications” (Agilent Technologies, publication 5991–2802EN, 2020).
(6) Agilent ICP-QQQ bibliography https://www.agilent.com/en/promotions/8900icp-qqq-trust.
(7) E. McCurdy, G. Woods, and N. Sugiyama, Spectroscopy 34(9s), 20–27,34 (2019).
Ed McCurdy and Michiko Yamanaka are with Agilent Technologies Inc. Direct correspondence to: ed_mccurdy@agilent.com ●

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Atomic Perspectives: Highlights from Recent Columns
March 3rd 2025“Atomic Perspectives,” provides tutorials and updates on new analytical atomic spectroscopy techniques in a broad range of applications, including environmental analysis, food and beverage analysis, and space exploration, to name a few. Here, we present a compilation of some of the most popular columns.