Liquid Chromatography–Atmospheric Pressure Photoionization-Mass Spectrometry Analysis of the Nonvolatile Precursors of Rancid Smell in Mayonnaise
Special Issues
For lipid-containing food products like mayonnaise, determining nonvolatile lipid oxidation products, the precursor compounds for rancidity, makes it possible to predict product shelf life at an earlier stage in product development. A method based on normal-phase liquid chromatography with atmospheric pressure photoionization-mass spectrometry (LC–APPI-MS) was developed for this purpose.
Monitoring lipid oxidation during the shelf life of lipid-containing food emulsions, such as mayonnaise, is challenging. It is, however, essential for the development of improved, consumer-preferred products. Determining the nonvolatile lipid oxidation products (NONVOLLOPS), the precursor compounds for rancidity, is required to determine the effectiveness of product stabilization technologies. A method based on normal-phase liquid chromatography with atmospheric pressure photoionization-mass spectrometry (LC–APPI-MS) was developed for this purpose. The inclusion of a size-exclusion chromatography (SEC) step was needed to remove interfering diacylglycerides and free fatty acids from the samples. The combined SEC and normal-phase LC–APPI-MS method allowed the identification of a wide range of oxidized species including hydroperoxides, oxo-2½ glycerides, epoxides, and other oxidized species. The method was found to be more suitable for the analysis of large sample sets. The relative levels of NONVOLLOPS from both ambient and accelerated stability tests could be determined. The results were compared to hexanal measurements. The data showed that NONVOLLOPS predict the rancidity of different formulations in a much earlier stage during shelf-life tests, providing valuable information for future product development.
The taste, flavor, and texture of lipid-rich foods, such as mayonnaises and dressings, strongly depend on the composition of the oil used and the emulsification methods applied during the preparation of the product. Making consumer-preferred products with healthier, unsaturated edible oils is a key challenge for the food industry. The unsaturated fatty acids in the triacylglycerols (TAGs) can undergo oxidation and hydrolysis reactions during the product's shelf life, forming volatile and nonvolatile lipid oxidation products. Even at very low levels the volatile lipid oxidation products can cause the typical rancid off-smell of aged oil. High amounts of nonvolatile lipid oxidation products could even adversely affect the consumer's health (1,2). Being able to accurately monitor lipid oxidation products is therefore crucial for safeguarding consumer safety and to study novel, natural stabilization technologies that suppress lipid oxidation reactions in lipid-rich foods.
A clear drawback of the healthier unsaturated fatty acids oleic, linoleic, and linolenic acid is their much higher amenability for oxidation reactions. The processes occurring during lipid oxidation so far are not fully understood, but it is believed that most reactions proceed through radical initiation (3). First, peroxidized and hydroperoxidized TAGs are formed that are precursors for the later volatiles. Dissociation reactions can then result in the loss of volatile compounds, such as aldehydes and ketones, and the formation of so-called "2½ glycerides." Other reaction pathways can lead to the formation of numerous nonvolatile oxidation products, such as hydroxy-TAG epoxides, TAGs with aldehyde functionalities, and polymers. Understanding the formation of these precursors is crucial for developing new stabilization technologies. The broad range of TAGs in edible oils combined with the wide variety of possible oxidation reactions makes the analysis of the nonvolatile precursors of the rancid smell very complex.
A number of fast and simple methods have been applied to monitor oxidation in oils. These include measuring the peroxide value (4,5) or the 2-thiobarituric acid value (6) of the oil. Although important and widely used, these measurements are highly empirical and unspecific (7). In addition, they do not really provide information down to the molecular level, which is needed to build a true understanding of the complex interplay of oxidation reactions. Chromatography combined with mass spectrometric detection is the method of choice for sensitive and selective analysis of both the volatile and nonvolatile intermediate and final lipid oxidation products. Volatile components are commonly measured by gas chromatography–mass spectrometry (GC–MS) headspace analysis (8). In the chromatographic analysis of nonvolatile oxidation products two factors must be considered. First, it should be realized that many of these products are not end products of the oxidation reaction, but are actually unstable intermediates. The analytical protocols applied for sample preparation and analysis should hence be very mild-and even then decomposition of labile intermediates might still occur. A second parameter to consider is the complexity of the mixtures obtained. Different classes of oxidized species are present, each consisting of a wide range of positional isomers and covering the entire chain length distribution.
We have demonstrated in previous work that normal-phase high performance liquid chromatography (HPLC) with electrospray MS detection gives detailed information on the nonvolatile lipid oxidation products present in oxidized oils (9). The method performs a polarity-based class-separation of the oxidized TAGs, providing information on groups such as the hydroxyperoxy-, hydroxyl-, epoxy-, or oxo (ketone or aldehyde)-TAGs, or TAGs with combinations of these chemical functionalities. This method remains challenging, primarily because of limitations in the repeatability and reproducibility. Sensitive electrospray ionization of the nonpolar compounds requires the use of a post-column additive to facilitate ionization, which unfortunately rapidly pollutes the ion source. Another limitation is the sample preparation. Normal-phase chromatography requires the samples to be free of water. Food products, such as mayonnaise and spreads, however, are emulsions containing up to 75% water. The products from the lipid oxidation reaction can accumulate in either the oil phase, the water phase, or at the oil–water interface. Freeze-drying effectively removes water, allowing extraction with organic solvents, but it is time consuming. Taking this all together, the normal-phase LC–electrospray MS method and the sample preparation required are far from ideal for studying the initial stages of lipid oxidation, especially if large sample sets have to be analyzed.
In this article, we describe a novel analytical approach for determining the nonvolatile oxidation products (NONVOLLOPS) in mayonnaise samples from (accelerated) shelf-life tests. The method uses atmospheric pressure photoionization (APPI) MS. The interpretation of the mass spectra is discussed and methods to distinguish MS fragmentation from overlap of nonreacted species are evaluated. Eventually, the nonvolatile precursors of the off-smell compounds of aged mayonnaise are determined. Understanding off-smell formation is built by correlating the results of the new NONVOLLOPS methods with those of classical hexanal measurements.
Experimental
Materials
Stable isotope labeled cholesterol D6 (purity 97–98%) was purchased from Cambridge Isotope Laboratories for use as an internal standard. Acetonitrile, isopropyl alcohol (IPA), and n-hexane (ULC–MS-grade) were obtained from Biosolve and tetrahydrofuran (HPLC-grade) was purchased from Merck.
Normal-Phase LC–APPI-MS
The HPLC system consisted of an Agilent 1200SL binary pump equipped with an HTC PAL autosampler (CTC Analytics), an Agilent 1200 series micro vacuum degasser, and an Agilent 1200 series thermostated column compartment. The mass spectrometer used was an Agilent 6410 triple-quadrupole MS system equipped with an Agilent G1971A APPI source. The ion source was equipped with a krypton discharge lamp and was operated in positive ionization mode. The optimized gas and vaporizer temperatures were 250 °C and 275 °C, respectively. The gas flow was 7 L/min, the nebulizer pressure was 40 psi, the capillary voltage was 2 kV, and the fragmentor voltage was 80 V. Full scan mass spectra were acquired from m/z 250 to 1000.
A 250 mm × 3.0 mm, 3-µm pursuit XRs 2 Diol column (Varian) was used for separation. The analytes were eluted at a flow rate of 0.3 mL/min using a linear gradient of n-hexane (Solvent A) and 9:1 (v/v) n-hexane–IPA (solvent B) programmed as follows: 5 min at 3% B, in 25 min to 8% B, in 10 min to 25% B, 10 min at 25% B, in 2 min to 50% B, 5 min at 50% B, in 1 min to 3% B, and finally 32 min at 3% B. The total run time was 90 min. The column temperature was maintained at 25 °C and the injection volume was 5 µL. The LC–MS method is not quantitative because of the lack of pure standards. In our method we used cholesterol-D6 as the internal standard enabling us to accurately establish (relative) concentration values.
Size-Exclusion Chromatography
The HPLC system consisted of a Shimadzu LC-10 isocratic pump equipped with an Optimas autosampler (Separations/Spark Holland), a GT-103 solvent degassing unit (Separations/Spark Holland), a Mistral column oven (Separations/Spark Holland), and a Shodex RI-71 refractive index detector (Shodex). Four 300 mm × 7.5 mm, 5-µm PLgel polystyrene–divinylbenzene size-exclusion chromatography (SEC) columns (Agilent) connected in series were used for the separation. The compounds were eluted at a flow rate of 0.8 mL/min using tetrahydrofuran as eluent at isocratic pump conditions. The column temperature was maintained at 30 °C and the injection volume was 10 µL. Chromeleon Chromatography software (Interscience) was used for data acquisition.
Volatile Compounds by GC
Hexanal measurements were performed according to AOCS recommended practice Cg 4-94 (10) on an Agilent 7890A GC–MS system equipped with a Gerstel MPS2XL multipurpose sampler.
Results and Discussion
The stability of various mayonnaise formulations was studied in a shelf-life test at ambient conditions over 200 days. The samples studied were mayonnaises obtained from local supermarkets and prototypes containing natural antioxidants (confidential stabilization technology 1 and 2). For each formulation tested and each time-point, 1 mL of sample was put in a 20-mL headspace vial. The vial was filled with air, capped, and placed in a temperature-controlled room (20 °C) in the dark. Samples were taken at seven-day intervals and were immediately stored at -80 °C to stop any further oxidation and awaited analysis as one large batch at the end of the shelf-life study. Preparation of the samples consisted of freeze-drying for 48 h. Water was removed under mild conditions and all species, irrespective of whether they were present in the water- or lipid-phase, or at the oil-droplet interface, were retained. After freeze-drying, 4 mL of n-hexane was added to the vials to dissolve the lipids and their oxidation products. Upon dissolution the proteins precipitated and clear sample solutions were obtained that could be directly injected into the normal-phase LC–APPI-MS instrument.
The normal-phase LC separation is largely based on polarity, with retention of the lipids and their oxidation products being determined by the presence of polar groups resulting from the oxidation reaction. The retention order of the oxidation products was established in our earlier studies (9). As an illustration, Figure 1 shows the normal-phase LC–APPI total ion current chromatogram of a 154-day aged mayonnaise after freeze-drying. The first eluting group are the unreacted TAG, which are eluted almost unretained. The next group is that of the epoxy- or oxo-TAG, oxidation products containing one additional oxygen as a ketone or aldehyde functionality. 2½ glycerides are slightly more polar and are eluted between 25 min and 40 min. In the 40-min to 50-min time interval, triglycerides with a single hydroperoxide group are eluted. More oxidized lipids are retained longer, with lipids containing two or more hydroperoxide groups eluting at retention times above 50 min.
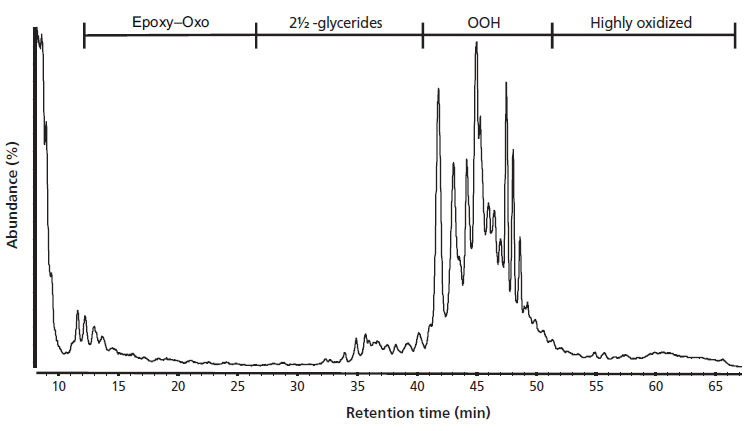
Figure 1: Lipid oxidation products in oil extracted from aged mayonnaise.
APPI is known for its efficient ionization of nonpolar or low-polarity compounds, such as sterols and their oxidation products (11). In APPI the ion source is less susceptible for pollution because no additives are needed to facilitate ionization as would be the case in electrospray ionization. In photoionization, two ionization pathways can be exploited: direct photoionization or dopant-assisted photoionization (12). In this work ionization of the compounds was achieved by APPI using the self-doping effect of the hexane-based mobile phase (13). The photons emitted by the krypton discharge lamp have energies in the range of 10 to 10.6 eV, which is above the ionization potential of the n-hexane eluent (10.18 eV). Via charge exchange and proton transfer, the primary hexane ions cause ionization of the analytes in the gas phase. The effectiveness of this approach is shown in Figure 2. This figure shows the mass spectra for some of the more abundant peaks in the time window from 40 to 50 min in Figure 1. All mass spectra show intense signals, especially in the mass range between m/z 850 and 900. From the LC elution positions it is likely that these signals represent hydroperoxides. The molecular ions of hydroperoxide-TAGs with three C18 fatty acyl chains would be expected at m/z values of approximately 920 Da, depending on the number of double bonds in the molecule. However, these molecular ions do not seem to be present. Mass losses of 18 and 34 Da are seen, indicating the loss of water or hydroperoxide groups and resulting in the fragment ions [M+H-H2O]+ or [M+H-H2O2]+, respectively. Smaller ions with m/z values in the 400 Da to 600 Da range are also seen in this retention time window. These could result from in-source fragmentation, but they could also represent other species of similar polarity and lower molecular weight. Experiments at other ionization settings (fragmentor voltage 50 V to 100 V) did not change the abundance of the 400–600 Da ions. It was concluded that these signals are not caused by fragmentation, but most likely represent other compounds. Clearly the chromatograms and MS spectra obtained are too complex for further interpretation. Additional separation of these complex samples could greatly aid the interpretation of the results obtained.
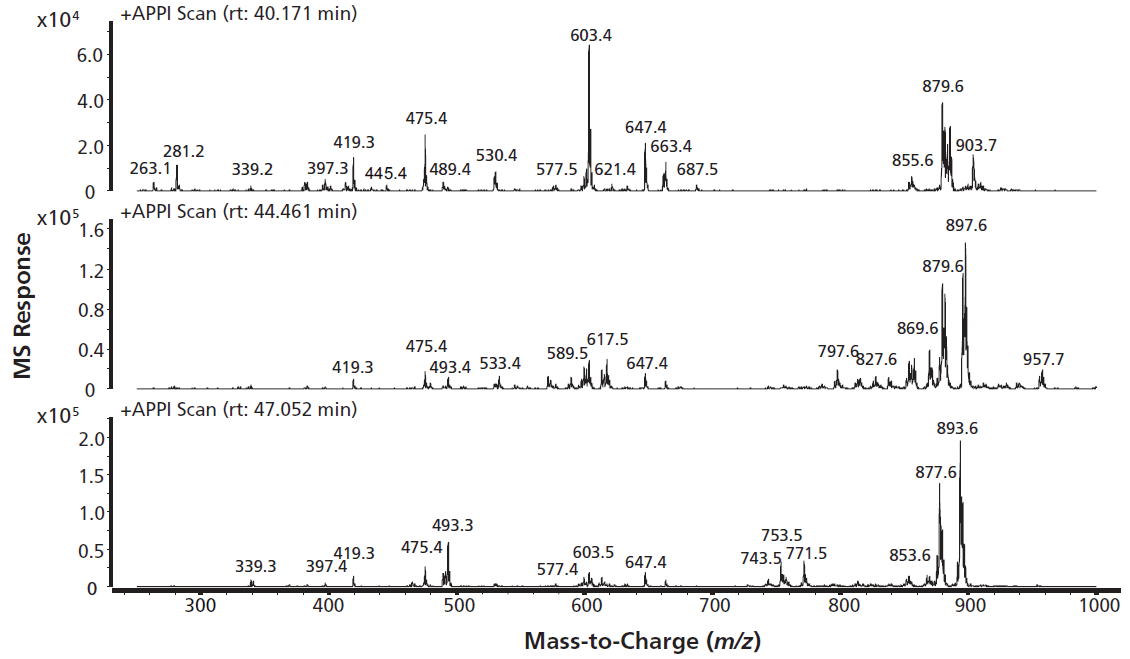
Figure 2: Mass spectra of multiple coeluted lipid oxidation products and neutral lipids.
In the initial normal-phase LC separations of the aged mayonnaise, multiple peaks were seen with polarities corresponding to one or more hydroperoxy-groups, yet molecular weights far below that of the HOO-TAG. SEC was applied to determine the molecular weight distribution of the sample, focusing in particular on the polymerized TAG, the TAG, as well as the di- and mono-acylglycerols and the free fatty acids. By using SEC with refractive index detection the relative content of each of these individual groups could be determined. A representative SEC chromatogram is shown in Figure 3. The largest peak represents the TAGs, or, more correctly phrased, TAGs and compounds with a molecular size similar to that of a TAG. This group accounts for 95.8% of the total signal. Primary oxidation products are just one or a few oxygen atoms larger than the TAG and hence in SEC are coeluted with the TAGs. Significant levels of diglycerides (DAGs) and free fatty acids (FFAs) were also seen in the SEC separation. These species are present at concentration levels of 2.4% and 0.7%, respectively, and could explain the low-molecular-weight polar species seen in normal-phase LC–APPI-MS. Furthermore, polymerized triglycerides are present at a concentration level of 1.1%. These are secondary oxidation products known to be formed from the fusion of two hydroperoxides via different pathways (14). The levels of DAGs and FFAs are high relative to those of the oxidized TAGs. They interfere with the TAG-oxide identification and quantification, especially in the analysis of samples from the initial stages of oxidation where the levels of the oxidation products are still low.
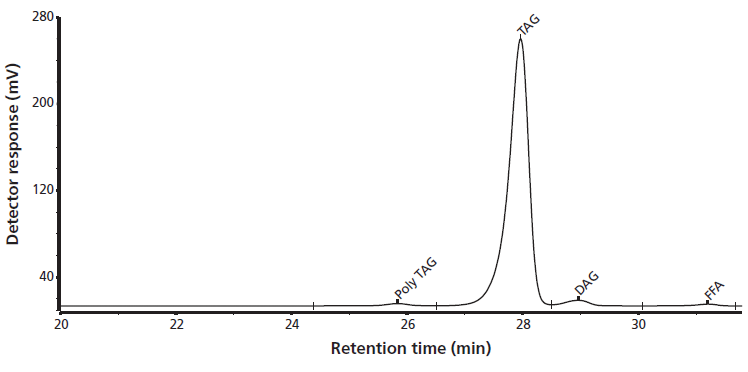
Figure 3: Determination of the relative content of polytri-, tri-, di-, monoacylglycerides, and free fatty acids by size-exclusion chromatography.
To eliminate overlap of DAGs and FFAs with the oxidized TAG in the normal-phase LC–APPI analysis, samples were prefractionated based on size by SEC prior to normal-phase LC analysis. Fractions of the eluent were collected in amber colored vials in four time windows: 29–33.5 min, poly-TAG window, 33.5–35.5 min, (oxidized-)TAG, 35.7–37.5 min, DAG, and 37.8–40 min, FFA. To obtain enough material, the procedure was repeated twice and the fractions were combined. After evaporation of the eluent under a stream of nitrogen, the fractions were redissolved in 100 µL n-hexane containing cholesterol D6 as internal standard before normal-phase LC–APPI-MS analysis.
The total ion current chromatograms of the size fractions containing the (oxidized-)TAGs and the DAGs are shown in Figure 4. Most of the polar compounds in both fractions are eluted between 40 min and 50 min. Note that the nonoxidized TAGs are eluted almost unretained in normal-phase LC and are not shown in these chromatograms. Hence, the peaks seen in the size fraction of the TAG are oxidized compounds with a size comparable to that of the TAG. The normal-phase LC–MS profiles of the two SEC fractions containing the (oxidized-)TAG or the smaller DAG are very different. Clearly, however, without SEC preseparation, these two compound groups of widely different size yet identical polarity would overlap. Six main peaks can be distinguished in the DAG fraction. The first eluted peak at 42 min has an m/z of 603.5, the second peak an m/z of 601.5 (43 min), and the third peak has an m/z 599.5 (44 min). These masses correspond to the [M+H-H2O]+ ions of the diacylglycerols OO, OL, and LL/OLn, respectively, where O represents oleic acid, L linoleic acid, and Ln linolenic acid. Positional isomers of these diacylglycerols also are eluted in the same retention order between 47 min and 49 min. From this it can be concluded that the low-molecular-weight peaks seen in the initial normal-phase LC separation without prior SEC separation were not representing oxidized TAGs, but rather partial hydrolysis products formed upon oxidation. The retention time differences between the different DAGs can be explained by the number of double bonds and the position of the free hydroxide group on the glycerol backbone. The last eluted peaks have a vacant outer position on the glycerol backbone.
The "triglyceride" SEC fraction contains the triglycerides next to the oxidized TAGs. The unreacted TAGs are unretained in normal-phase LC and are not shown in Figure 4. More-polar species, such as the epoxy-TAGs and the hydroperoxy-TAGs, are eluted in the retention time windows of 10–25 min and 40–50 min. The interferences of the unreacted DAG are now removed meaning that the mass spectra are easier to interpret. In Figure 5, MS spectra are shown of the epoxy–oxo lipids, hydroperoxides, 2½ glycerides, and the highly oxidized lipids. The MS spectra of some important classes of lipid oxidation products are discussed in detail below.
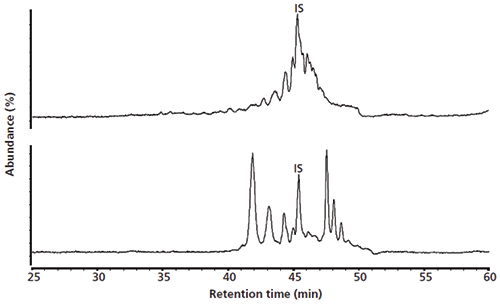
Figure 4: Normal-phase LC-MS analysis of fractions isolated using size-exclusion chromatography. Top: Fraction of TAGs and compounds of similar molecular size. Bottom: Fraction of DAGs and compounds with similar molecular size.
For the hydroperoxides, protonated molecules [M+H]+ are visible, for example at m/z 913.7, at a relatively low abundance. The most prominent peaks are m/z 897.7 and 883.7, and are formed by the loss of water giving [M+H-H2O]+, or the loss of a hydroperoxide group, leading to [M+H-H2O2]+ ions. The DAG fragment ion m/z 603.5 is formed after loss of the fatty acyl chain with the hydroperoxide functionality. Another characteristic DAG fragment ion is visible at m/z 619.6. During ionization water is lost and an epoxide group is formed across the site of unsaturation converting it to a chain with no unsaturation (OS-epoxide) (14). All TAGs with two adjacent O fatty acids can form this fragment ion. In the rape seed oil used to prepare the mayonnaises analyzed here the primary TAGs showing this behavior are OOLn, OOO, and POO. The fragment ion at m/z 493.4 is formed from breakage of this epoxide bond, resulting in the loss of a C9H18 group.
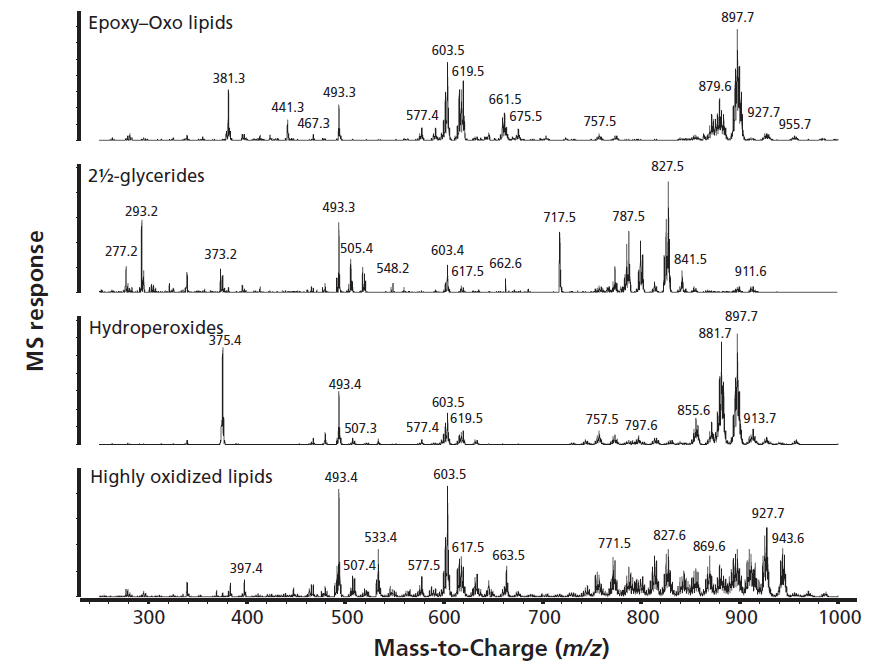
Figure 5: Mass spectra of lipid oxidation products after removal of abundant diacylglycerols.
The earlier eluted epoxides yield mainly protonated molecular ions, with an example of this being m/z 897.7. The mass spectrum is nearly identical to that of the hydroperoxides, except that there is no mass loss of 34 Da visible (loss of H2O2).
The 2½ glycerides oxidation products, the remaining nonvolatile part of the triglyceride after chain-scission and release of the volatile off-smell, are secondary oxidation products formed from breakdown of the hydroperoxides. They are present in the DAG fraction, but not clearly visible in the TIC chromatogram. Their abundance is low in comparison to the signal of the DAGs. The structures with 2½ fatty acid chains are actually oxo-2½ glycerides, with molecular masses corresponding to that of the parent triglyceride (for example, LLO) after the loss of a hexanal molecule (C6H12O mass 100 Da). It is important to note that the oxo-2½ glycerides group is very complex. The original molecules can contain different fatty acids from which different volatiles can be split off. Moreover, the remaining fatty acid chains can be oxidized at one or more additional positions. In the extracted ion chromatograms, the oxo-2½ glycerides are detectable in the samples. The oxo-2½ glycerides with an additional oxidation group are eluted later from the column and do not interfere with the analysis.
As the degree of oxidation increases and TAG with multiple oxygens incorporated in their structure are formed, the mass spectra become even more complex. Possible structures are epoxyhydroperoxides, dihydroperoxides, dihydroxyperoxides, and cyclic peroxides. In the mass spectra multiple losses of 18 and 34 Da are visible, suggesting the loss of water and hydroperoxide groups upon ionization. Full interpretation of these compounds remains difficult, however.
The SEC fractionation was applied on a limited number of samples to build understanding of the MS spectra by eliminating overlapping compounds with similar polarity but different sizes. The combined results of the chromatographic and MS spectral evaluation of the different lipid oxidation groups are presented in Table I. The retention time windows and the m/z values used to generate extracted ion chromatograms to zoom in on specific oxidation products can be seen. Examples of the molecular structures of the oxidized species are also given in the table. Specific positions of the polar oxidation groups on the fatty acid chains can vary depending on the original location of the double bonds. Note that the combination of retention times and spectral information is unique. This means that not all samples have to go through the time-consuming SEC prefractionation. Analyzing a few samples after DAG removal is sufficient to establish the time windows and MS traces of interest.
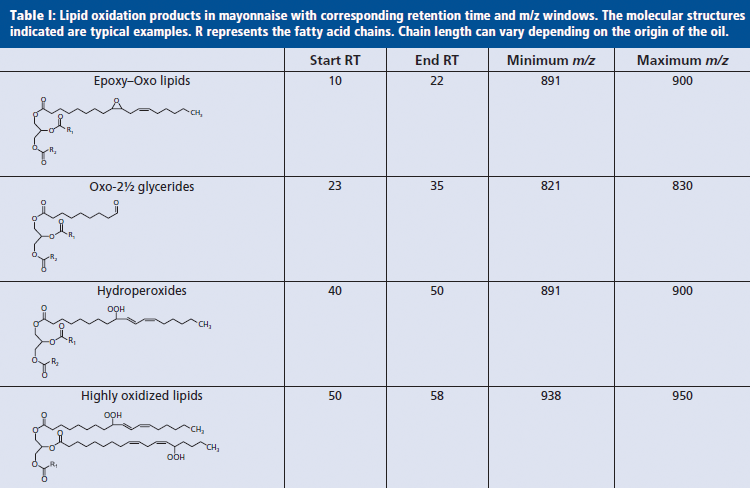
To meet the clear trend in society for more natural foods, the food industry is trying to replace non-natural stabilization strategies by natural preservation routes. To this end formulations with differences in the applied stabilization technology were tested in a large-scale storage test. The main goal of this study was to find suitable replacements for EDTA (E385, E386) and understand how the different stabilization routes determine the oxidation reactions and the levels and types of oxidation products. Figure 6 shows the response plots of the nonvolatile species determined by normal-phase LC–APPI-MS. Over 150 samples could be analyzed without maintenance. From this perspective the newly developed APPI-MS method performed much better than our previously described ESI approach (9). Parallel to the LC–MS measurements sensory testing was performed. Hexanal measurements were also performed, but the levels were too low for the classical static headspace method. Clear trends are visible in the plotted data, and the levels of all monitored oxidation markers increase with storage time. Moreover, differences between the applied stabilization technologies are visible as well. The formulation with EDTA (positive control) appears to function best in delaying oxidation by showing the lowest increase in NONVOLLOPS over time. When no stabilization technique was applied (negative control), the oxidation products increased the most rapidly. The proprietary stabilization technology 2 performs better in this shelf-life test than technology 1: formation of the secondary oxidation products oxo-2½ glycerides and highly oxidized lipids occurs at a later time-point in the shelf-life test and the levels are lower. Interestingly, hydroperoxides seem to reach a constant plateau level, more or less identical for each of the formulations tested. The possibility that this phenomena was caused by a nonlinear response of the MS detector was ruled out by measurement of several diluted samples yielding similar curves. The occurrence of a plateau suggests that the formation of hydroperoxides at some point comes to a halt, or that the rate of breakdown and formation become equal. The latter explanation here is much more logical. The hydroperoxides are unstable intermediates that can be converted into other volatile and nonvolatile products. This is supported by the steady increase of oxo-2½ glycerides, degradation products of the hydroperoxides, over time. Attempts to correlate the occurrence of nonvolatile oxidation products in this ambient shelf-life trial with the release of hexanal failed because of an insufficient sensitivity of the classical GC–MS headspace method.
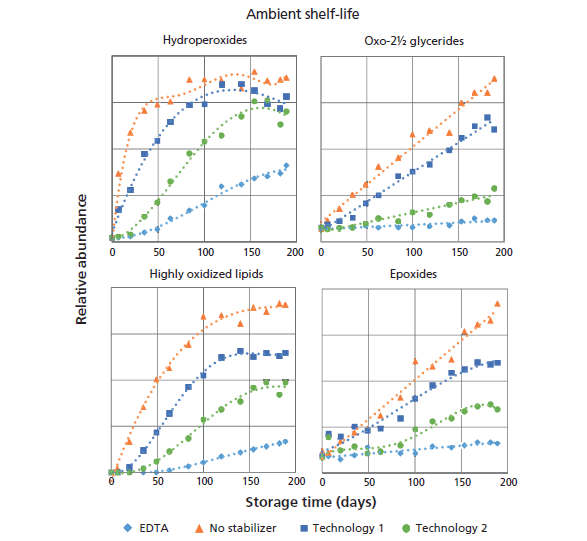
Figure 6: Trend lines of nonvolatile lipid oxidation markers measured during an ambient shelf-life test. Different lines represent different stabilization technologies.
To enable correlation of NONVOLLOPS levels with hexanal release, an additional (accelerated) shelf-life test was performed at 50 °C with a sampling interval of three days. The effect of the increased temperature on the formation of volatile and nonvolatile oxidation products is shown in Figure 7. GC–MS was applied to measure hexanal (10). The SEC method originally set up to remove interfering species was applied to monitor the formation of polymerized TAG.
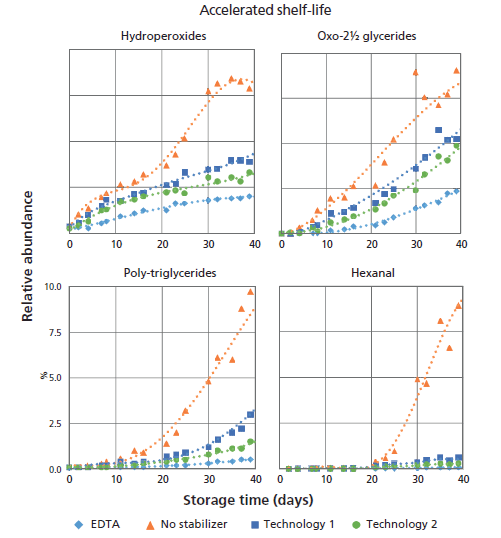
Figure 7: Trend lines of volatile and nonvolatile oxidation markers measured during a 50 °C accelerated shelf-life test. Different lines represent different stabilization technologies.
At the 50 °C elevated shelf life temperature substantial oxidation occurred. After 40 days of storage, the SEC measurements show poly-triglycerides at up to 10% of the total lipid content. In the GC–MS experiments hexanal was clearly detectable. When looking at the time curves of the hydroperoxides and oxo-2½ glycerides a trend similar to that seen in the ambient study is observed, albeit that the oxo-2½ glycerides are detectable much earlier in the 50 °C storage test. Already after 11 days this marker is detectable and clear differences between the formulations become apparent. This differentiation is not so clear from the results of the hexanal and poly-TAG analyses in the initial stages of the oxidation process. Both hexanal and poly-TAGs show a clear lag phase. Initially they are hardly formed, but after a certain lag phase their levels start to increase at a much faster rate than those of the oxo-2½ glycerides. From this observation, we postulate that the oxo-2½ glycerides may undergo further breakdown and are, like the hydroperoxides, not end products of the oxidation reaction. Whether they are lost by conversion to poly-TAG or oxidize further by the additional formation of hydroperoxide or epoxide groups on the remaining unreacted fatty acid chains cannot be derived from the current data. This concept of continuous oxidation with intermediates and end products is schematically illustrated in Figure 8. In this "oxidation engine," triglycerides and oxygen are the starting products that form intermediate oxidation products. These products may undergo a continuous process of oxidation and breakdown and eventually end up as volatiles and polymerized lipids in the samples. Clearly the reactions occurring in lipid oxidation are more complex than the "oxidation engine" suggests (3), yet the principle of the engine clearly illustrates the processes occurring. Stabilization technologies control the initial rate of formation of hydroperoxides, as well as the speed of the engine in a way still largely not understood. The new methods presented here shed some light on the oxidation reactions and products forming, and can help in making the search for alternative stabilization technologies to protect lipid-rich foods from oxidation less of a trial-and-error exercise.
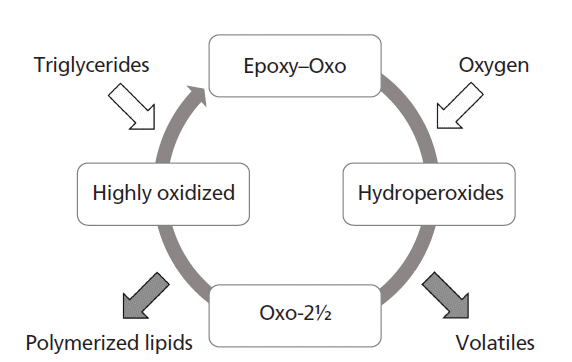
Figure 8: Schematic representation of the "oxidation engine," a continuous process where volatile, nonvolatile, and oxidation end products are formed through routes with different intermediates.
Conclusions
The newly developed normal-phase LC–APPI-MS method allows the detection of nonvolatile lipid oxidation products in the initial and later stages of lipid oxidation. With the detailed information on the NONVOLLOPS, we were able to determine the efficiency of different stabilization technologies in complex food emulsions. This was achieved by successful ionization of NONVOLLOPS using the self-dopant effect of the mobile phase. Detailed interpretation of MS spectra was possible using pre-separation by SEC. The relative levels of important NONVOLLOPS such as hydroperoxides, oxo-2½ glycerides, and epoxides could be determined in large sample sets from ambient and accelerated stability tests. It is envisaged that the developed method will play an important role in developing new, natural stabilization technologies to suppress lipid oxidation in lipid-rich foods.
Acknowledgments
The authors thank Justin van 't Veer for performing the GC–MS hexanal analysis.
References
(1) G. Billek, Eur. J. Lipid Sci. Technol. 102(8–9), 587–593 (2000).
(2) J. Kanner, Mol. Nutr. Food Res. 51(9), 1094–1101 (2007).
(3) H. Yin, L. Xu, and N.A. Porter, Chem. Rev. 111(10), 5944–5972 (2011).
(4) M. Hicks and J.M. Gebicki, Anal. Biochem. 99(2), 249–253 (1979).
(5) A. Lips, R.A. Chapman, and W.D. McFarlane, Oil Soap 20(11), 240–243 (1943).
(6) H. Ohkawa, N. Ohishi, and K. Yagi, Anal. Biochem. 95(2), 351–358 (1979).
(7) B. Barriuso, I. Astiasarán, and D. Ansorena, Eur. Food Res. Technol. 236(1), 1–15 (2013).
(8) S. Panseri, S. Soncin, L.M. Chiesa, and P.A. Biondi, Food Chem. 127(2), 886–889 (2011).
(9) L. Steenhorst-Slikkerveer, A. Louter, H.-G. Janssen, and C. Bauer-Plank, J. Am. Oil Chem. Soc. 77(8), 837–845 (2000).
(10) AOCS Recommended Practice Cg 4-94. Volatiles (VOC) in Fats and Oils by GLC. Official Methods and Recommended Practices of the AOCS, (American Oil Chemists Society, Champaign, Illinois, 1998).
(11) C.H. Grün and S. Besseau, J. Chromatogr. A 1439, 74–81 (2016).
(12) D.B. Robb, T.R. Covey, and A.P. Bruins, Anal. Chem. 72(15), 3653–3659 (2000).
(13) T. Ghislain, P. Faure, and R. Michels, J. Am. Soc. Mass Spectrom. 23(3), 530–536 (2012).
(14) E.N. Frankel, J. Am. Oil Chem. Soc. 61(12), 1908–1917 (1984).
Boudewijn Hollebrands, MSc, completed his master study in analytical sciences at the University of Amsterdam in 2013. Currently, he is a research scientist in the analytical department of Unilever R&D Vlaardingen, the Netherlands. His work focuses on the development of hyphenated chromatographic techniques and high-resolution mass spectrometry methods for food and nutritional analysis.
Hans-Gerd Janssen is science leader of chromatography and mass spectrometry at Unilever R&D Vlaardingen. He also is a part-time professor in biomacromolecular analysis at the University of Amsterdam, Amsterdam, the Netherlands. Professor Janssen's research interests include the development of multidimensional separation systems for food, biomedical, and environmental analysis.

Best of the Week: AI and IoT for Pollution Monitoring, High Speed Laser MS
April 25th 2025Top articles published this week include a preview of our upcoming content series for National Space Day, a news story about air quality monitoring, and an announcement from Metrohm about their new Midwest office.
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.