Mass Spectrometry for First Responders
Mass spectrometry is now among the tools used by first responders to identify potential threats, but there are challenges involved, including interpreting results and ensuring data relevance.

First responder (FR) teams cultivate traditional expertise in handling hazardous chemical materials, often responding to incidents at sites containing known and labeled materials. Rapid unknown chemical threat identification, as in response to a possible terror threat, is a newer challenge for these teams. The technological challenges are substantial, but not insurmountable, and the tools used for analysis and identification in the field now include mass spectrometry (MS). This column provides some perspective about issues involved in FR field analysis of chemical threats.
Make the following statement as you walk into any top-notch analytical laboratory with a collection of sophisticated mass spectrometers and a cast of experts: "I need the answer in 10 minutes." Then show them a sealed evidence bag containing an envelope coated with, and spilling out, white powder. Ask what it is and whether it's an immediate cause for personal concern, or concern for persons located in a much larger physical area where the envelope was found. Make it known that you will not go away until you get the answer.
Welcome to a maybe not-so-uncommon scenario in the world of the first responder (FR). It is unlikely that anyone will show up at a general-purpose analytical laboratory entirely unannounced with such an evidence bag. As a result of the need for prompt analyses, there are some analytical labs that practice for such a situation, and have the processes and instrumentation in place that expedite an accurate analysis. But there can only realistically be so many of these laboratories distributed across a geographical area. The acute need for rapid, and sometimes on-site, analysis of chemical threats found at incident sites is real and growing, and the consequences of decisions made with the analytical data on-hand are significant. The luxury of adequate time to decide on a sampling and analysis protocol, to replicate results, and to quantify results is often absent. Sometimes, knowledge and expertise is developed in response to a particular incident. The anthrax-typing capability used in a recent U.S. case is an example of analytical development occurring after the initial incident and the FR action. We can study our ability to respond, but ultimately, an FR analysis uses the tools at hand, works to interpret the available data, and then someone uses the data to make a decision. The quality and the completeness of the data inform the quality of the decision. The value of data lies in its immediacy and its applicability, and an incorrect answer can be as detrimental as no answer at all.
How MS Can Be Used with First Responders
Let's assume that mass spectrometry (MS) can make a contribution to the analytical data sought by first responders. It certainly seems to be a safe assumption because there are a number of vendors with instruments developed to meet that market need. Although funding for purchase and operator certification and training is sometimes available, these instruments may be on the more expensive end of what's available. There's usually room for a small mass spectrometer and the power to operate it in a standard-size emergency response vehicle. With smaller instruments, robots and rovers can bring a portable mass spectrometer physically close to almost any FR site. Handheld instruments can go anywhere the FR goes.
A search for "portable mass spectrometer" reveals early publications more than 60 years old (1–3). Even back then, it was clear that to make the design of the mass spectrometer smaller and more robust was a solvable issue of applied engineering and clever design, and that sampling was a key issue in usability. In work published by J.J. Buckley and colleagues (3), the researchers designed a sampling capillary that drew an air sample from within the lung and provided near real-time readings of carbon dioxide levels in expired breath. It is worth noting that the sampling capillary was 40-ft long (3), a distance that allowed the mass spectrometer to be safely sited outside of the operating room, but still allowed for lung gas analysis of carbon dioxide in real time.
In an overview of mass spectrometers developed for first responders, it may seem at first that portability would be the major criterion. With the 60-year history of portable mass spectrometers, the list of transportable and mobile instruments has grown long. Literature reviews describe developments and potential uses (4–7). The construction of portable instruments based on previously unexploited mass analysis principles continues to be developed (8). Miniaturization of instrumentation leads to smaller footprints, lower power requirements, and the capability for placement in unique environments, such as in spacecrafts or in underwater locations, as described recently in a column in this series, and certainly within the FR area of application. The analytical challenges of the FR application is perhaps the most demanding of all. It is vitally important that the instruments actually be deployed and tested in the field, with as close to real conditions as possible (see reference 9 for a recent example), recognizing the fundamental fact that no single instrument-based analysis (no matter what the vendor may claim) is likely to provide definitive analytical threat identification. Furthermore, programs are underway (with their own unique technical challenges) to integrate the instrumental output from MS systems with other sensors (that may include chemical, biological, radioactive, and explosives detectors) into an automated network that displays the broader spatial picture of a dispersed threat. The broad overview would be used by informed decision makers to calibrate a response (10). Specialists will then discuss the process of integration of the MS data with all other data, but a bit of reserve in recognizing that MS is not the first and end-all approach to FR analysis would be wise.
Making mass spectrometers small enough to be portable and integrating the MS data into an automated network for decision making are only two of the scaling parameters that define the value of MS in an FR environment. But, as recognized by Buckley and colleagues (3) more than 60 years ago, another incredibly important parameter is sampling. It is self-evident that FRs are confronted with the task of sampling a substance of unknown quality. That substance may be explosive or toxic, and handling may make it more so or disperse it. Sampling in the classical sense usually involves sample handling — collection, dissolution, perhaps derivatization, and finally, injection of an aliquot of sample into the mass spectrometer. None of these actions is appealing to FR experts, who, quite understandably, might be attracted to "no-touch sampling." No-touch sampling includes desorption electrospray ionization (DESI) and direct analysis in real time (DART) technologies (11,12), both of which offer novel ways of sampling substances and surfaces. The sensitivity of these sampling methods is sufficient for practical FR use. The real-world threat level from an explosive or toxic compound can be orders of magnitude higher than the demonstrated limits of detection using no-touch sampling. Further, these sampling methods allow (with sufficient time) a spatial distribution of potentially toxic compounds to be generated. DESI and DART are described as variants of "ambient ionization mass spectrometry," and it is this new approach to sampling that will make significant headway into FR mass spectrometry in the future.
Which MS Systems Meet FR Needs?
Figure 1 represents the conceptual balance between the three aspects of instrumental MS important for FR discussed above. Smaller physical size for instruments makes them more attractive for FR use, whether slightly larger instruments are cached on a first responder vehicle or the device becomes so small that it can be handheld. Data sharing is represented by the second vector, with mass spectral data acquired rapidly and then uploaded into a network for integration and support of decision-making. Again, the value of mass spectral data rises when the data are combined with other data in attempts to identify an unknown substance. Finally, sample handling is the third vector, with the central nexus representing the idealized "no-touch sampling" exemplified by DART and DESI.
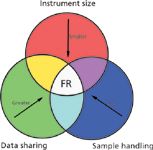
Figure 1: Three performance-related issues (instrument size, data sharing, and sample handling) and the vectors that lead to a nexus of applicability for the first responder (FR).
A technology survey model has been created for instruments that meet FR needs, reflecting the broad need for analysis of chemical, biological, and radiological agents. The model uses a technology readiness level (TRL) descriptive rating that spans from 1 to 9. Level 1 designates a technology for which the basic concepts have been established. Levels 4 and 5 mean that a first-generation device has been constructed and is operational in a laboratory setting. Levels 6 and 7 mean that the device technology has been brought into the field. Level 9 means that the technology has been successful in mission deployment. The TRL represents a model that follows a logical progression of technology development. The pathway is not always so rationally illuminated or accurately forecast. As McGee (13) writes: "Unknown powders, hazardous liquids, and gases are now routinely tested on-site using a wide range of portable instruments. The testing is usually performed by highly trained specialized teams, but not always, since portable instruments are marketed as easy to use, even for operators without a scientific background. However, many portable instruments are not good at identifying chemical mixtures despite the marketing claims."
Issues with Technology Surveys
Clearly then, there must be other elements (or finer related details) relevant to FR application for MS that may be difficult to capture within a TRL model. An overarching consideration of what is known, what is not known, and what you can learn about either, is part of the joy of being an analytical chemist (14). So, to wrap up this column, let's drill down to some perspective of the analytical chemist in an FR problem. Time is the first issue we consider, as exemplified by the 10-min scenario described at the start of this column. Time is not explicitly captured within the "data sharing" vector of Figure 1, which assumes that data are more or less created instantaneously by any sensor. For mass spectrometers, it is true that data are captured within a few seconds at most, with some time added for chromatographic separation if used. However, it's the interpretation of the data that always requires additional time. For example, contrast an immediate need for evaluated data with the far more leisurely exposure to an unknown exemplified by a mass spectral interpretation class. As both student and teacher of such classes, I can testify to the stress of uncertainty, and the extended time used, in attempting to identify an "unknown" from its electron ionization mass spectra, as an example. Reaching logical dead ends in the interpretation, the student begins to question all assumptions — whether the putative molecular ion is that highest mass ion, or maybe it doesn't appear because a facile initial fragmentation reduces its relative intensity. Students think to themselves: "Maybe the spectrum isn't even of a pure sample, and the given mass spectrum represents two, three, or even more separate compounds. Or maybe the compounds are related, or maybe some sample degradation has occurred." The time is ticking away, and some answer has to be written down for the test. "Maybe I'll get some partial credit, and still pass the course." There's no partial credit in the real world — but there will surely be a paucity of time. Simply put, it is not realistic for FR personnel to be experts in spectral interpretation, and there is no reasonable expectation that 10 min is sufficient time for even an expert to "interpret" data.
McGee (13) explains that the idea of placing portable equipment in the hands of specialized law enforcement teams or frontline police officers is appealing, but questions whether it is realistic or not to turn police officers into chemists. "Placing sophisticated instruments that detect hazardous chemicals into the hands of law enforcement personnel is not without challenges and concerns," states McGee. He continues by explaining that laboratory analysts usually have enough time to run enough tests to unambiguously identify a chemical or a mixture of chemicals, fully evaluate the results, confer with colleagues, consult the scientific literature, or repeat the tests. "In contrast, the field analyst is expected to generate results quickly, then interpret and explain them so that the decision maker can use the information to confidently make immediate decisions," says McGee. McGee goes on to explain that determining whether the white powder is cocaine or a homemade peroxide-based explosive can be a matter of life or death in certain circumstances and may require a building's mass evacuation if the substance is an explosive. According to McGee, "Lab-quality data may or may not be required on-site. But field operators must understand their instruments' capabilities and limitations so they can produce reliable, useful, and defensible results."
McGee's suggestion in the last sentence may reach too far. Field operators cannot realistically be responsible for the defensibility of the results, at least as far as analytical relevance is concerned. As noted earlier, FR personnel may decide on and defend the sampling and the collection of data. The details of measurement are linked to instrument design and interpretation is linked to how the data are processed. The FR does not design the instrument. Within 10 min in the FR world, it is inevitable that mass spectral data can only be matched to a standard within a database, or not, and matching will be done by software rather than by personal analysis, and thus this is again not within FR control. My assessment here seems to be at odds with the fact that classic mass spectral interpretation is taught within some curricula aimed specifically at FR personnel, and I conclude that curricular hours would be better spent teaching sampling. The TRL scale evaluation may determine whether recorded data are sufficient for identification of a sample in a simulation, and a higher rating is achieved when it can be shown that the real-world data are relevant and useful. But it yields no clue as to how identification is achieved or about the potential roadblocks and hurdles that determine success or failure. The rating may lack an analytical anchor.
Data Relevance
Another issue that deserves some expansive description is data relevance, but it is not captured in Figure 1. Although time is a metric that commercial vendors can benchmark, at least in simulation, relevance is a more difficult and much more important issue. Relevance is established in real-world tests and is difficult to create and difficult to manage. But it's part of the joy of being an analytical chemist that you can ponder what you know, what you don't know, and how it all fits together (14). For FR analysis, consider what France (15) wrote: "During a recent investigation of letters containing a white powder sent to a targeted company in several states, a common field detection device failed to identify the white powder." France explained that the FRs used a device to identify the white powder from one of the letters in the field but their instrument gave them a result that a nontoxic, inert chemical was present. The responders sent a sample to a public health laboratory for testing and the laboratory later identified the powder as a toxic chemical that could potentially cause illness if inhaled or ingested. France concluded: "The incident was a reminder to other first responders, though, to be constantly aware of the limitations of their own field-detection devices." We set up simulations of real-world testing based on experience and what common threats might be, based on what has been identified as a threat before. The result of this approach to evaluation is that any white powder is viewed both with suspicion and preconceived notions, leading talc and cornstarch to instill concern by appearance alone. What if a toxic (usually white) powder was dyed blue or green? Would the apparent color confound the analytical result?
As France notes, the limitations of field-detection devices should indeed be acknowledged, but many of those limitations relate to the data produced based on the operation method chosen. The issue of data relevance itself includes grand scale issues such as whether data are sufficient for identification or to rule out an identification (which may be just as valuable). The relevance test for data includes a knowledge of inter-relationships between data derived from different instruments or sensors, and knowledge of what data are independent and what data are duplicative. The relevance test means that contradictory data are not discarded or ignored, but collected and used along with any other data. The urge to discard data that do not fit a presumed hypothesis is ever present, and it is insidious. The relevance of FR data is often compared against the data held in a database that corresponds to known or previously identified threats. France describes a laboratory response network (LRN) that helps to assess the relevance of data by pooling expertise and minimizing weaknesses due to inexperience or local bias. But as always, time may be needed to access that expertise, and 10 min may be too short a time to query the network in the usual manner.
Conclusions
So finally, this analytical chemist notes our tendency in creating portable mass spectrometers for FR has been to create smaller instruments interfaced to smaller data systems that process and interpret the data on-site, and present a result to the FR. We have simply miniaturized the scale, but we maintain the same way of performing the analysis. It may be useful to rethink that approach. Improved communications (cell phone–based data links, augmented by evolving suitcase cell towers) make it possible to move surprising amounts of data locally, nationally, and if needed, globally. The databases and the heuristic software to reach decisions need not reside on-site, but can be located anywhere, along with expert staff on call. The capability for instruments, mass spectrometers included, to upload data into a 24/7 cloud network, is likely to grow increasingly important. Stay tuned.
References
(1) J.A. Hipple, Nature 150, 111–112 (1942).
(2) F.A. Miller, A. Hemingway, A.O. Nier, R.T. Knight, E.B. Brown, Jr., and R.L. Varco, J. Thoracic Surg. 20, 714–728 (1950).
(3) J.J. Buckley, F.H. VanBergen, A. Hemingway, H.L. Demorest, F.A. Miller, R.T. Knight, and R.L. Varco, Anesthesiology 13(5), 455–464 (1952).
(4) C.M. Henry, Anal. Chem. 71(7), 264A–268A (1999).
(5) E.R. Badman and R.G. Cooks, J. Mass Spectrom. 35, 659–671 (2000).
(6) C.M. Henry, Chem. Eng. News 80(14), 34–35 (2002).
(7) Z. Ouyang, R.J. Noll, and R.G. Cooks, Anal. Chem. 81, 2421–2425 (2009).
(8) D.L. Hood, M.S. thesis, Ohio University, 2009. Available at: http://etd.ohiolink.edu/send-pdf.cgi/Hood%20Derrell%20L.pdf?ohiou1248981763.
(9) J.A. Contreras, J.A. Murray, S.E. Tolley, J.L. Oliphant, H.D. Tolley, S.A. Lammert, E.D. Lee, D.W. Later, E.D. Lee, and M.L. Lee. J. Amer. Soc. Mass Spectrom. 19, 1425–1434 (2008).
(10) S.E. George, "S&T Chemical and Biological Defense Programs," presented at the DHS Symposium on Bioterrorism Risk, October 6, 2009.
(11) C.C. Mulligan, N. Talaty, and R.G. Cooks, Chem. Commun. 1709–1711 (2006).
(12) J.M. Wells, M.J. Roth, A.D. Keil, J.W. Grossenbacher, D.R. Justes, G.E. Patterson, and D.J. Barket, Jr., J. Amer. Soc. Mass Spectrom. 19, 1419–1424 (2008).
(13) E. McGee, The Police Chief 76(9), September 2009.
(14) C.G. Enke and L.J. Nagels, Anal. Chem. 83(7), 2539–2546 (2011).
(15) R. France, "The Field Testing Dilemma and LRN Chemical Laboratories," a commentary appearing January 14, 2009 in Domestic Preparedness. See www.domesticpreparedness.com.
Kenneth L. Busch has taught the short course "Introduction to Mass Spectrometry" for 18 years, and the class usually includes a few first responders. The stories told from their experiences are enlightening and a little bit scary, and they help define the analytical balance between what we know and what we don't know. An important quality-determining step for mass spectral data is always the sampling end of the process. In the old days, we called this GIGO. Nowadays, there's probably an app for that. This column is the sole responsibility of the author, who can be reached at WyvernAssoc@yahoo.com.

Kenneth L. Busch

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.