New Developments in Wavelength-Dispersive XRF and XRD for the Analysis of Foodstuffs and Pharmaceutical Materials
Spectroscopy
September 2006. The authors discuss the benefits of X-ray fluorescence (XRF) for the determination of elemental nutrients in foodstuffs and X-ray diffraction (XRD) for the measurement and characterization of different compounds used in the pharmaceuticals industry.

Over the past 30 years, X-ray fluorescence (XRF) has become the elemental technique of choice for the determination of parts-per-million to percentage concentration levels in many solid materials including metals, ores, rocks, glasses, powders, plastics, ceramics, and foodstuffs (1). The principles of XRF spectrometry are well-documented in the literature (2). A sample is irradiated with a beam of high-energy X-rays. As the excited electrons in the sample fall back to a ground state, they emit X-rays that are characteristic of those elements present in the sample. The individual X-ray wavelengths are then separated and measured via a system of crystals, optics, and detectors. Elemental concentrations in unknown samples are quantified by comparing the X-ray intensities against known calibration standards. The major benefit of XRF over arc or spark emission techniques is that it can analyze both conducting and nonconducting materials as well as inorganic and organic matrices with minimal sample preparation (3,4).
X-Ray Fluorescence
The technique is available in two separate configurations — energy-dispersive (ED) and wavelength-dispersive (WD) XRF. The ED approach measures the intensity of the photon energy of the individual X-rays generated, whereas in WD spectrometry, the polychromatic beam emerging from a sample surface is dispersed into its monochromatic components or wavelengths with an analyzing crystal. A specific wavelength is then calculated from knowledge of the crystal and the diffraction angle (5).
It is generally recognized that WDXRF has several advantages over EDXRF, including better spectral resolution (up to 10 times better for some elements), superior performance for light elements (B to Cl), and the ability to determine major concentrations of elements without risking saturation of the detectors. Figure 1 shows the periodic table, indicating all the elements that can be determined by WDXRF.

Figure 1: Elements that can be determined by WDXRF.
The quality of the accuracy and precision of the data generated by WDXRF can very well be compared with that of traditional wet chemical methods, but with the clear advantage of faster analysis times. This translates into a significant reduction in the overall cost of analysis, as no complex, labor-intensive wet chemistry procedures are required. The other major benefit of the WDXRF technique is that in addition to conventional quantitative analysis using suitable calibration standards, the technique is able to analyze various types of materials in which no matching standards are available, or where the calibration procedures are time-consuming and expensive. This feature has become possible thanks to the development of powerful "standard-less" analysis software programs using stored calibration graphs. It is generally accepted that the higher performance and better capabilities of WDXRF are helping to push the analytical boundaries traditionally associated with the XRF technique.
X-Ray Diffraction
On the other hand, X-ray diffraction (XRD) is an analytical technique that looks at the X-ray scattering from crystalline or polymorphic materials. Each material produces a unique X-ray "fingerprint" of X-ray intensity versus scattering angle that is characteristic of its crystalline atomic structure. Qualitative analysis is possible by comparing the XRD pattern of an unknown material with a library of known patterns. Although its principles are different, XRD can be considered complementary to XRF. For example, XRF can tell you that a material is composed of iron and sulfur, but XRD can tell you that both iron sulfide (FeS) and elemental iron (Fe) are present. Furthermore, because XRD works with any crystalline solid, there is almost no limit to the types of materials that can be studied (6). Some of the materials that are typically characterized by XRD include chemicals, environmental dusts, rocks, minerals, metals, cements, pigments, and forensic samples.
One of the major applications of XRD is to identify and quantify specific compounds or phases in materials that are organic in nature, such as foodstuffs or pharmaceutical compounds. These kinds of applications have evolved over the last few years mainly because of a new generation of solid-state detectors, higher quality optics, and the availability of sampling attachments. These technological improvements have led to both higher sensitivity, which is needed for the detection of minor and trace levels of crystalline compounds in pharmaceutical compounds, and higher resolution for improved spectral specificity in the presence of interfering species.
To demonstrate the benefits of both the WDXRF and XRD techniques, let us take a closer look at some of the more challenging applications that are being carried out today in the pharmaceutical and food industries.
Application of WDXRF to the Analysis of Foodstuffs
WDXRF has been widely used for the chemical analysis of foodstuffs, pharmaceutical raw materials, and agricultural crops (7,8). Standard element coverage is from sodium (Na) to uranium (U), with typical concentration levels from sub-part-per-million levels up to 100%. However, lighter elements (Be to O) can be determined in certain matrices with the use of synthetic multilayer crystals. Sample preparation is very simple. Sometimes, the sample can be analyzed as it is received, or the more common procedure is to dry the sample, grind it into a powder, and then press it into a pellet or tablet. The XRF data shown in this study were generated either on the low-powered (50-W) ARL OPTIM'X or the medium-powered (1.2-kW and 2.5-kW) ADVANT'X, (Thermo Electron SA, Ecublens, Switzerland) WD-XRF spectrometers. They both have been described in the open literature (9). A schematic of the ADVANT'X showing the principles of sequential operation is shown in Figure 2.
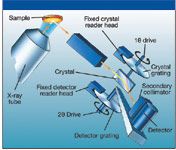
Figure 2: Schematic of the WDXRF instrument used in this study.
Analysis of Milk Powders
The analysis of infant cereals and milk powders is becoming an increasingly important application for XRF (7). However, one of its greatest challenges has been to determine the low-level nutrients like Se, Mn, Fe, and Zn with good precision and accuracy. For that reason, a comparison of the limits of detection (LOD) in ppm was made on a series of pelletized milk powders using three different excitation sources. This comparison is shown in Table I. The quantitative detection limits are similar for all three approaches, but there is a definite trend toward lower detection limits as the power is increased. Typical measurement precision values (ppm) for six separate analyses of the same pellet (using low power) are shown in the last two columns of the table. However, it is important to emphasize that even though this kind of precision is considered typical for low-level XRF work, the accuracy of the measurements depends on many other factors, including operator experience, sample preparation, calibration standards, and whether appropriate background correction is used.

Table I: Comparison of limits of detection (LODs) of a high (up to 4.2 kW), medium (1.2 kW), and low (50 W) power WDXRF system for the analysis of pelletized milk powders (t = measurement/integration time; NM = not measured) (Note: All precision and LOD date are ppm)
Nitrogen in Plant Material
Another example of the progress made in the capability of XRF to detect very low atomic numbers is shown in Figure 3, which shows a spectral scan of nitrogen in plant material. Ten years ago, nitrogen belonged to the list of the elements that could not be determined by WDXRF. Advances in multilayer crystal technology, the thickness and material of detector windows, and instrument sensitivity now allow nitrogen to be included in the list of elements that can be determined by XRF.
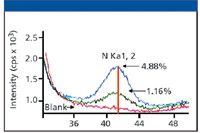
Figure 3: A spectral scan of nitrogen in plant material obtained using a high-power WDXRF system.
This is exemplified in Figure 4, which shows the nitrogen calibration curve obtained for various plant materials (tomato, hay, cabbage, lettuce, wheat, grass, leaves) using the same instrument.
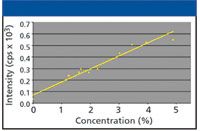
Figure 4: Calibration of nitrogen using various plant materials.
Application of X-Ray Diffraction to the Analysis of Pharmaceutical Materials
Let us now turn our attention to X-ray diffraction. The new generation of X-ray diffractometers has stimulated the growth of the technique in the pharmaceutical and food industries both for research and development purposes and for routine quality control testing (10). For example, crystallographic, structural, and phase analysis can give pharmaceutical companies more complete characterization of new materials. However, in such applications, it is absolutely critical that the instrument offers high sensitivity combined with extremely good resolution down to low angles.
Stability of pharmaceutical formulations as a function of relative humidity also can be tested using specially designed environmental chambers. To ensure that formulations will be stable under all possible storage conditions, it is important to know how the material reacts to changes in temperature. In particular, it is possible to know whether thermal expansion is isotropic (identical in all directions) or not (anisotropic). If a given formulation has preferred orientation and anisotropic thermal expansion, changes in storage temperature can result in tablet breakage or other formulation problems. For these kinds of manufacturing quality control applications, it is therefore important that in addition to high performance, the instrument also is fitted with suitable sampling accessories.
With that in mind, let us look at some typical XRD applications that are being carried out in the pharmaceutical industry. The XRD data shown in this study were generated using an ARL X'TRA powder X-ray diffractometer (Thermo Electron SA) (11). A schematic of the instrument's geometry is shown in Figure 5.
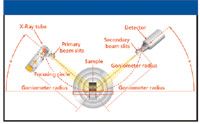
Figure 5: Schematic of the X-ray diffractometers used in this study showing the θ-θ geometry.
Measurement of Crystalline Phases
The design of this instrument makes it very suitable for the identification of polymorphic (crystalline) and pseudo-polymorphic compounds generally encountered in pharmaceutical and food compounds (12). Thanks to its high sensitivity, low concentration levels of polymorphic phases can be detected more easily. This can be seen in Figure 6, which shows a 1-h diffraction scan of X-ray intensity against scattering angle of a drug compound. The boxed area in green has been expanded to show the detection of trace levels of a secondary phase of the polymorph. This phase of the compound (ranitidine hydrochloride) was then quantified by standard calibration methods based on peak area measurements shown in Figure 7.

Figure 6: Detection of a polymorphic compound present at trace levels (

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.