A Novel Method for Practical Implementation of the Shifted Excitation Raman Difference Spectroscopy (SERDS)
Special Issues
SERDS analysis can be easily and successfully carried out using two high-power laser diodes with a fixed-wavelength separation in the presence of high fluorescence.
Shifted-excitation Raman difference spectroscopy (SERDS) has been demonstrated to be effective in situations where high fluorescence prevents effective use of Raman spectroscopy. Here, we describe a novel way to apply SERDS using pairs of high-power laser diodes with a fixed-wavelength separation. This approach was used to carry out quantitative Raman analysis of liquid mixtures in the presence of high levels of fluorescence as well as qualitative analysis of several naturally fluorescing compounds. Also, the use of a simple spectrum reconstruction algorithm is compared with the conventional method of removing the fluorescence background via numerical background fitting.
There is growing interest in the use of Raman spectroscopy in a vast scope of applications in various industries, including pharmaceuticals (1–5), petrochemicals (6–8) and law enforcement (9–13). This growth is driven in part by increased offerings of portable and affordable Raman instruments, which are powered by compact, high-performance wavelength-stabilized laser diodes. However, as the scope of applications for Raman spectroscopy broadens, the challenge of sample fluorescence arises more frequently. Besides the natural fluorescence that many substances possess, samples can also become contaminated with fluorescent compounds in the field (9,10). An example is that of fluorescent agents such as caffeine and flour regularly used to cut illicit street drugs (10).
To combat sample fluorescence, laser excitation at longer wavelengths has long been used (14–16). Indeed, if it were not for sample fluorescence, nearly all Raman spectroscopy could be performed with the same short-wavelength laser, especially because blue and violet diode lasers are becoming more and more powerful and affordable. For substances with weak short-infrared (IR) fluorescence, 785-nm and 830-nm laser excitation have been an acceptable compromise. For samples exhibiting stronger short-IR fluorescence, one must resort to 1064-nm excitation.
However, using 1064-nm excitation presents a number of issues for portable field instrumentation. First, it is well known that the Raman signal diminishes as the fourth power of laser wavelength, which means that it is 16 times weaker with 1064-nm excitation as it is with commonly used 532-nm excitation. At the same time, it is not always possible to compensate for a decreased Raman scattering cross-section by increasing laser power, because a stronger laser may damage the sample. As a result, collection times that are nearly an order of magnitude longer would be necessary to collect as much Raman signal when using a 1064-nm laser. Furthermore, when a laser of this wavelength is used for conventional Raman spectroscopy, the Raman signal appears at wavelengths well outside the sensitivity range of silicon detectors as a result of Stokes shift, which makes it necessary to use an InGaAs detector instead of a silicon charge-coupled device (CCD). This presents an additional problem, because the dark noise of an InGaAs array is more than an order of magnitude worse than that of the Si CCDs. As a result, InGaAs detector arrays for dispersive Raman instruments need to be cooled to achieve satisfactory noise levels, and even then they don't reach the performance of CCDs operating at room temperature without cooling. This situation presents an obvious problem for portable, battery-operated Raman instrumentation, because it means more power consumption, a larger battery, and shorter battery life, not to mention that it increases the cost and complexity of the instrument. Another common approach to performing Raman analysis with 1064-nm excitation is Fourier transform Raman (FT-Raman). This approach simplifies detection by using a single, discrete cooled InGaAs or Ge detector, but at the same time significantly increases the complexity of the optical system, because it requires a stable Fabri-Perot interferometer to perform the analysis. FT-Raman systems tend to be larger and more expensive and consume more power than a dispersive Raman instrument that uses an uncooled CCD detector would.
For these reasons, the use of shifted-excitation Raman difference spectroscopy (SERDS) has long been considered attractive, effectively expanding the use of conventional dispersive Raman instrumentation equipped with efficient CCD detectors to the classes of samples that exhibit fluorescence. Historically, the SERDS method has been used in laboratories employing tunable-wavelength lasers. However, this type of laser source presents a significant challenge for portable Raman systems. Generally, these lasers are much more costly than simple and efficient wavelength-stabilized laser diodes. In addition, there are challenges in ensuring the exact wavelength control over these lasers required to perform accurate qualitative and especially quantitative Raman analysis. To ensure accurate analysis, the wavelength must be monitored constantly.
We have studied a comparatively simple and practical approach to performing SERDS analysis using two affordable wavelength-stabilized laser diodes operating at slightly offset wavelengths. The lasers are stabilized by use of volume Bragg gratings (VBGs) and are compact and efficient, making them well suited for portable battery-operated Raman instrumentation.
Experimental
The experiments were performed using an LS-2 dual-laser SERDS laser source (PD-LD, Inc.). The lasers operated at the 784.5-nm and 785.5-nm wavelengths. The wavelength separation was selected to correspond to the approximate line width of the Raman lines of the substances under study. Note that the exact wavelength separation is not significant for the accuracy and practicality of SERDS; however, wavelength separation that is much smaller than the width of the Raman bands would result in increased noise in the SERDS spectra.
The LS-2 laser source delivers the output of either one of the lasers to the output port through a fiber-optic switch. A fiber-optic Raman probe was attached to the output port of the laser source and thus delivered light to the samples under study. The same probe collected the Raman signal in the back-reflection geometry, together with fluorescence and the Rayleigh scattering. The filters installed in the fiber-optic probe suppressed the Rayleigh scattering so that the collected Raman scattering signal and the fluorescence were delivered to the input port of the spectrometer via the collection fiber in the probe.
For these experiments, we used a compact spectrometer with an uncooled CCD array (model USB2000+, Ocean Optics). The spectrometer had a 50-µm slit and approximately 10-cm-1 resolution. For SERDS analysis, the spectra were collected sequentially, first with laser 1 and then with laser 2. The lasers ran continuously during the course of the experiment to ensure best stability of the output power. The wavelengths of the lasers were stable to <5 pm over the course of the day. The samples under study were in either liquid or solid form. A majority of the samples (usually liquids) were placed in small glass vials and illuminated through the bottom of the vial using the fiber-optic probe.

Figure 1: Normalized raw Raman spectra of R6G dye in water solution collected at two different laser excitation wavelengths.
After collection, the spectra were stored for further processing. The spectra were corrected for the dark background and the relative pixel response (the white light correction) and normalized. After that, the difference spectra were calculated for SERDS analysis. For conventional Raman analysis, the spectra were fitted with a polynomial (a fourth- or sixth-order polynomial was used) and the polynomial background was then subtracted from the original spectrum. In addition, a numerical derivative of the original spectrum was sometimes calculated for performing some of the quantitative Raman analysis.
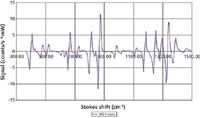
Figure 2: SERDS difference spectrum of the R6G dye in water solution. The spectrum was obtained with the data shown in Figure 1.
Both SERDS spectra and the Raman spectra processed by conventional methods were compared with the library spectra for identification. Note that in no case was any a priori knowledge used for either identification or quantitative Raman analysis. Once identified, the spectra were also processed for quantitative determination of the relative concentration of the two major identified constituents, as is discussed below in more detail.
Results
To illustrate what collecting Raman spectra at the two SERDS wavelengths achieved, we show the spectra of R6G dye dissolved in water (Figure 1). As one can easily see, Raman peaks shift together with the laser wavelength, whereas the fluorescence background does not. Figure 2 shows the difference spectrum produced from the spectra shown in Figure 1. It is easy to see that the spectrum is very clean and free from any residual features of the fluorescence. Note also that SERDS spectrum automatically removes any etalon effects on the CCD.

Figure 3: Raw, unnormalized spectra of a binary mixture of methanol and ethanol with strong fluorescence background from the R6G dye. The spectra shown were collected at the two SERDS fixed excitation wavelengths.
Given that SERDS spectra are free from any background, have zero integral over the spectral window, and have a nearly perfect derivative shape, they are extremely convenient for performing Raman analysis, as they are nearly orthogonal for the majority of the substances of interest for Raman analysis. To illustrate this point, we performed quantitative Raman analysis on binary mixtures of methanol, ethanol, isopropanol, and acetone in the presence of a strong fluorescence background. In this experiment, the obtained SERDS spectrum was compared with library SERDS spectra, the components of the mixtures were identified, and then the relative concentration of the two most likely components in the mixture was determined. No multivariate calibration methods (such as partial least squares regression) were used. Thus, the system was not trained on the known mixtures; all samples were treated as completely unknown.
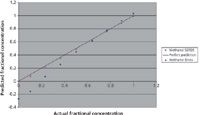
Figure 4: Prediction of the relative concentrations of the two alcohols, methanol and ethanol, obtained in the presence of a strong fluorescence background from the R6G dye. The amount of fluorescence in the samples was the same as is shown in Figure 3. The purple squares show prediction based on SERDS analysis. The blue diamonds show prediction based on a conventional numerical processing method (calculating the derivative numerically).
To compare the results obtained by SERDS with what conventional Raman analysis would predict, the fluorescence background was removed via numerical derivatization and then an analysis similar to the SERDS analysis was carried out. The Raman spectra were collected for a series of solutions with different concentrations of methanol in the presence of ethanol. All solutions contained a large amount of R6G dye to simulate strong interference from fluorescence. The raw Raman spectra of one of these solutions is shown in Figure 3. Figure 4 shows the predicted concentrations of methanol in the mixtures obtained via SERDS and the conventional method of numerical derivatization. As one can see, although both methods give reasonably accurate predictions of the methanol concentration when it is present in large proportions, the picture is quite different when a lower proportion of the methanol is present in the mixture. The conventional method starts to lose accuracy at a concentration of about 20–25% and then fails completely at concentrations lower than 10%. SERDS, on the other hand, predicts accurate concentrations even at 5% methanol content in the mixture. Note that in these experiments the ratio of Raman signal to fluorescence background was about 1:200, so that Raman peaks were barely observable over the fluorescence.

Figure 5: Raw Raman spectrum of dark Jamaican rum. Strong fluorescence masks any sign of the ethanol Raman peaks.
The above example of quantitative Raman analysis clearly illustrates the power of the SERDS methodology as compared with conventional methods. In addition, SERDS also can be useful for simpler tasks of identification of a compound that is naturally fluorescent or is masked by fluorescence from an unwanted additive. In such situations, identification should also be carried out using the differential SERDS spectra, without reconstruction. Quite often, however, there is a need for a human operator to take a look at the obtained spectra for additional control. In those cases, the derivative shape of the SERDS spectrum is undesirable, because it is not easily recognizable by a human. To assist in this task, reconstruction of the proper Raman spectrum from the differential SERDS spectrum must be carried out. This can be easily accomplished using a simple interpolation + integration algorithm described in an article by Matousek and colleagues (17). All the reconstructed spectra that appear in this article have been obtained using that algorithm.
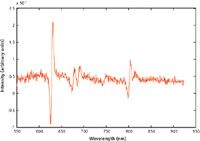
Figure 6: SERDS difference spectrum of the rum obtained from the raw Raman spectrum shown in Figure 5. The derivative shape of the Raman spectrum of ethanol is very clearly defined.
To illustrate the power of SERDS for this type of application, we analyzed strongly fluorescent Jamaican dark rum, which is often used as a benchmark in Raman analysis of fluorescent samples. Figure 5 shows a raw Raman spectrum of the dark rum. As can be easily noticed, essentially no Raman features are observable in this spectrum, justifying its use as a benchmark substance. Figure 6 shows the SERDS spectrum of the rum, where derivative-shape Raman features of ethanol are easily identifiable. The reconstructed spectrum of the dark rum is shown in Figure 7 in comparison with the conventional Raman spectrum obtained via polynomial baseline subtraction. There are no Raman peaks observable in the conventional spectrum, whereas a trained eye can easily recognize the Raman features of ethanol in the spectrum reconstructed from SERDS.
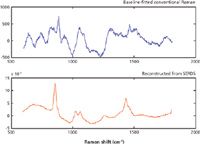
Figure 7: Raman spectra of the dark rum: reconstruction based on SERDS data shown in Figure 6 (red line), and the conventional Raman spectrum obtained with polynomial baseline fitting (blue line).
Besides rum, which is used as a benchmark material but presents little interest for most other purposes, there are a number of substances that must be quantitatively and qualitatively analyzed for legitimate purposes. As an example, Figure 8 shows normalized Raman spectra of a regular olive oil and an extra virgin olive oil. The extra virgin olive oil is clearly more fluorescent and could be distinguished by just that, but analysis of the Raman features provides much more useful information. The Raman spectra of both oils reconstructed from SERDS are shown in Figure 9. The differences in Raman features between the two oils are subtle, and proper multivariate analysis is required for this classification task.

Figure 8: Normalized conventional Raman spectra of two olive oils: regular oil (blue line) and extra virgin olive oil (red line).
Discussion
The results presented above demonstrate the advantages of SERDS over conventional Raman methods. These advantages stem from the accurate elimination of the fluorescence contribution, as opposed to approximating it by some smoothly varying polynomial. Another advantage of the method is its simple and accurate elimination of the stationary noise in the signal in the process of spectra subtraction. The only source of noise that is not eliminated is the shot noise associated with fluorescence. Nevertheless, it is easy to reduce shot noise by averaging over a larger number of collected spectra. Note that because exposure times need to be shortened to avoid saturation of the CCD when the fluorescence is strong, increasing the number of averages may not even result in much longer times for completing the analysis. It is instructive to observe that an increase in the number of averaged spectra does not lead to better results in the case of the conventional method of baseline fitting for fluorescence removal.
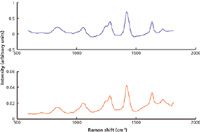
Figure 9: Raman spectra of the two olive oils reconstructed from SERDS difference spectra. Blue line shows regular olive oil and the red line the extra virgin olive oil.
Conclusion
We have shown that SERDS analysis can be easily and successfully carried out using two VBG-stabilized laser diodes operating at two slightly offset fixed wavelengths. The method has been shown to provide accurate measurement of the concentration of a minor constituent in a binary liquid mixture of alcohols at 5% of the minor component in the presence of a strongly fluorescence background that exceeded the Raman signal by a ratio of approximately 200:1. Any conventional Raman analysis carried out under the same conditions with the same excitation wavelength would fail at a concentration approximately three times larger of the same component in the mixture. The analysis was performed without any a priori knowledge about the substances in the sample; no prior training was performed on the system using any of the multivariate analysis methods.
To illustrate the power of the approach compared with the conventional method of the polynomial background subtraction, we have analyzed dark rum and several other naturally fluorescent compounds. From this analysis it is evident that the conventional method cannot recognize or extract the spectrum of ethanol from the rum regardless of the number of averages taken on the original spectra. By contrast, SERDS gives a clear identification of ethanol and allows a reasonably clean reconstruction of the ethanol spectrum, which improves proportionately to the number of averages.
Therefore, the SERDS method performed in the fashion described here is a practical and viable solution for Raman analysis in situations where a strongly fluorescent background is present. The solution is especially suitable for portable Raman systems where size, cost, and power consumption are of primary importance. It allows quick, effective, and affordable Raman analysis in the majority of real-life situations, thus obviating the need for more expensive, bulky, and power-hungry 1064-nm Raman systems.
B. Appiah, S. Dolgy, V.S. Ban, E.D. Melnik and B.L. Volodin are with PD-LD Inc. in Pennington, New Jersey. B. Appiah is also with Princeton University, in Princeton, New Jersey. Direct correspondence to bvolodin@PD-LD.com.
References
(1) C. Gendrin, Y. Roggo, and C. Collet, J. Pharm. Biomed. Anal. 48, 533 (2008).
(2) S. Wartewig and R.H.H. Neubert, Adv. Drug Deliv. Rev. 57, 1144 (2005).
(3) L.S. Taylor and F.W. Langkilde J. Pharm. Sci. 89, 1342 (2000).
(4) W.P. Findlay and D.E. Bugay, J. Pharm. Biomed. Anal. 16, 921 (1998).
(5) D. Pratiwi, J.P. Fawcett, and K.C. Gordon, T. Rades, Eur. J. Pharm. Biopharm. 54, 337 (2002).
(6) W.M. Chung, Q. Wang, U. Sezerman, and R.H. Clarke, Appl. Spectrosc. 45, 1527 (1991).
(7) J.B. Cooper, P.E. Flecher, T.M. Vess, and W.T. Welch, Appl. Spectrosc. 49, 586 (1995).
(8) M. Ku and H. Chung, Appl. Spectrosc. 53, 557 (1999).
(9) R.J. Stokes, K. Faulds, and W.E. Smith, Proc. SPIE 6741, 67410Q1 (2007).
(10) R.E. Littleford, P. Matousek, M. Towrie, A.W. Parker, G. Dent, R.J. Lacey, and W.E. Smith, Analyst 12, 505 (2004).
(11) E. Horvath, J. Mink, and J. Kristof, Mikrochim. Acta 14, 745 (1997)
(12) K. Faulds, W.E. Smith, D. Graham, and R.J. Lacey, Analyst 127, 282 (2002).
(13) B. Saegmuller, G. Brehm, and S. Schneider, Appl. Spectrosc. 54, 1849 (2000).
(14) S. Kaminaka, H. Yamazaki, T. Ito, E. Kohda, and H.-o. Hamaguchi, J. Raman Spectrosc. 32, 139 (2001).
(15) C.G. Zimba, V.M. Hallmark, J.W. Swalten, and J.F. Rabolt, Appl. Spectrosc. 41, 721 (1987).
(16) C. Engert, A. Materny, and W. Kiefer. Chem. Phys. Lett. 198, 395 (1992).
(17) P. Matousek, M. Towrie, and A.W. Parker, Appl. Spectrosc. 59, 848–851 (2005).

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.