Organic Nitrogen Compounds II: Primary Amines
Spectroscopy
Amines, which contain carbon, hydrogen, and nitrogen, come in six varieties, but using the N-H stretching peak positions by themselves will distinguish between all the different amine types.
In the second installment in our survey of the infrared spectroscopy of organic nitrogen compounds, we look at amines. These molecules contain carbon, hydrogen, and nitrogen. They come in six varieties, and, by using the N-H stretching peak positions by themselves, we can distinguish between all the different amine types. The Infrared Spectral Interpretation Challenge also returns after its holiday hiatus.
Many years ago, the American Chemical Society issued a series of bumper stickers with bad chemical puns on them. My favorite, given my background, was "Honk if you passed P-Chem." Another was, "It takes Alkynes to make a world." But the one most relevant to this column was, "It's Amine Old World without Chemists." It would be a mean world without chemists, or amines for that matter. Amines comprise a part of amino acids, from which proteins are made. It is hard to imagine life on earth without these molecules.
The amine functional group contains carbon, hydrogen, and nitrogen, and consists of C-H and C-N bonds. Amines are divided into three categories, called primary, secondary, and tertiary amines. Their molecular structures are seen in Figure 1.
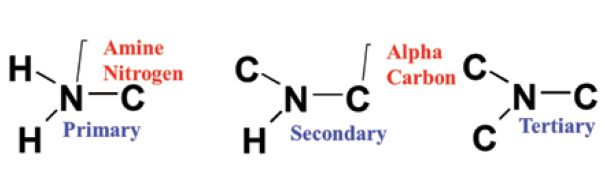
Figure 1: The molecular structure of primary, secondary, and tertiary amines. Note the locations of the amine nitrogen and alpha carbon.
Note that amines consist of a central nitrogen atom, called an amine nitrogen, single-bonded to various numbers of carbons and hydrogens. Any carbon atom attached directly to the nitrogen is called an "alpha carbon." The amine group structural names count the number of carbons attached to the amine nitrogen. Thus, a primary amine contains one C-N bond, a secondary amine contains two C-N bonds, and a tertiary amine contains three C-N bonds. Note that as the number of C-N bonds goes up, the number of N-H bonds goes down. Thus, a primary amine contains two N-H bonds, a secondary amine contains one N-H bond, and a tertiary amine contains no N-H bonds. This can be a little confusing, but as long as you remember that the amine names are counting the number of carbons attached to the amine nitrogen (and not the number of hydrogens), you will be all right.
The nature of the alpha carbons in an amine plays a role in determining the type of amine. If one or more of the alpha carbons are saturated in the functional group, it is called a saturated amine. If one or more of the alpha carbons are aromatic, then the moiety is called an aromatic amine. But what happens when one alpha carbon is saturated and the other is aromatic, as is the case for the molecule N-methylaniline seen in Figure 2?
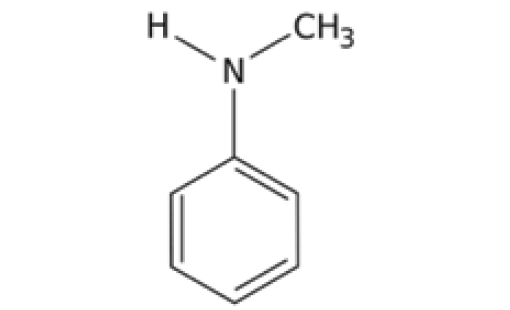
Figure 2: The chemical structure of N-methylaniline, an aromatic amine.
The answer is that aromaticity wins. If any of the alpha carbons in an amine are aromatic, the amine is considered aromatic. Thus, N-methylaniline is an aromatic amine. Ultimately, then, there are six different kinds of amines: primary saturated, primary aromatic, secondary saturated, secondary aromatic, tertiary saturated, and tertiary aromatic. Note it takes not one but two adjectives to properly describe an amine. All these types of amines may be bewildering, but, as we will see below and in future columns, infrared spectroscopy can be used to distinguish all these different types of amines from each other.
Recall from the last column (1) that organic nitrogen atoms form somewhat polar N-H bonds, and that these functional groups can form hydrogen bonds. The hydrogen bonding of amine groups to each other is seen in Figure 3.
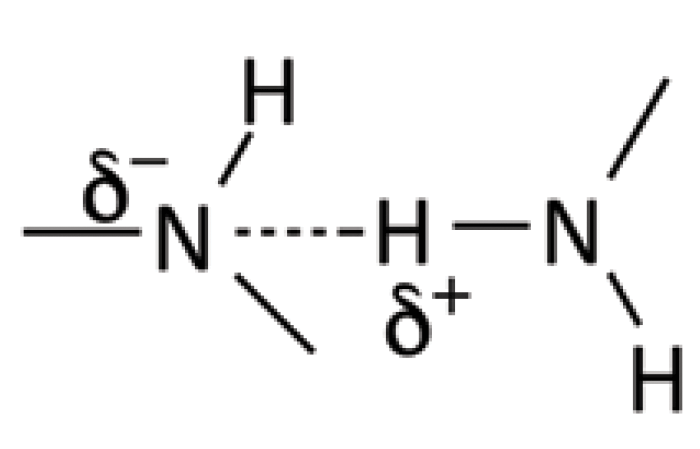
Figure 3: An illustration of the hydrogen bonding that takes place in amines.
Because of electronegativity differences, the nitrogen carries a partial negative charge, the hydrogen a partial positive charge, and they coordinate with each other as shown.
The Infrared Spectroscopy of Primary Amines
As we discussed last time (1), it is N-H stretching and bending peaks that are your best guide to identifying the presence of nitrogen in an organic compound. The primary amine-NH2 group has structural similarity to the methylene or CH2 group we have studied previously (2), and to water. In all cases, there is a central atom with two hydrogens attached, and all three atoms are in the same plane. Of course, in the case of the methylene group, the central atom is a carbon; for water, the central atom is an oxygen, and, for a primary amine, the central atom is a nitrogen. One difference between these molecules is the bond angles. In a methylene group, it is 109 °; in water, they are about 104.5 °, and, in a primary amine, the bond angles are 120 ° (3).
Primary amines have the stretching and bending vibrations seen in Figure 4. Like a methylene group and water, a primary amine has two stretching vibrations, an asymmetric and symmetric stretch. Note that, for the asymmetric stretch, the hydrogens move in opposite directions, with one hydrogen moving towards the nitrogen while the other moves away. For the symmetric stretch, however, both hydrogens move together in the same direction, either towards or away from the nitrogen. For both methylene, water, and primary amine groups, the number of spectroscopically useful hydride stretching vibrations matches the number of hydrogens attached to the central atom. In all cases, this is two. As we will see in the next column, an easy way to distinguish primary and secondary amines is that primary amines contain two N-H bonds, and hence have two N-H stretching peaks, whereas secondary amines have only one N-H bond, and only one N-H stretching peak. The NH2 group has a scissors bending vibration where the two hydrogens bend towards or away from each other, as seen in Figure 4. Primary amines also have an out-of-plane bend or "wagging vibration," where the two N-H bonds bend together above and below the plane of the page.
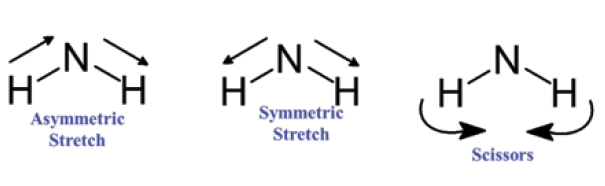
Figure 4: Vibrations of the primary amine group.
The spectrum of a primary amine, propylamine, is seen in Figure 5. The two N-H stretching peaks labeled A and B are the indication that this is a primary amine.
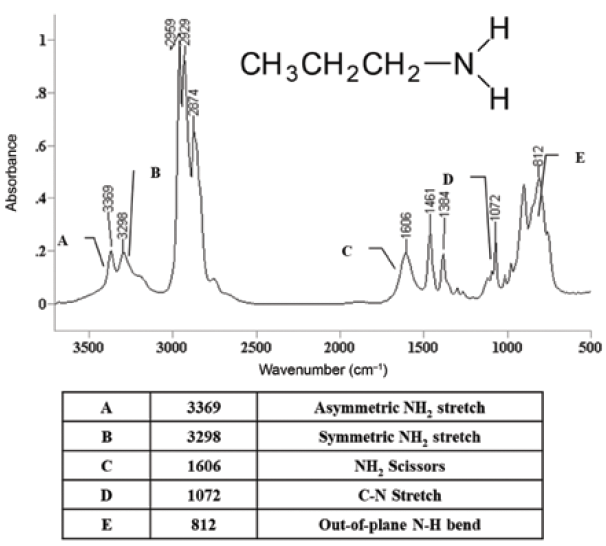
Figure 5: The infrared spectrum of propylamine (wavenumber positions shown within table).
Recall from last time (1) that N-H stretches fall in the same wavenumber range as O-H stretches, but that N-H stretches are easy to distinguish, because they will be less broad and less intense. This is due to N-H bonds being less polar than O-H bonds (1). The N-H stretches of propylamine fall at 3369 cm-1, which is assigned as the asymmetric stretch, and 3298 cm-1, which is the symmetric stretch (going forward, assume all peak positions are in cm-1 units). Note that the peaks are somewhat broadened, a couple hundred wavenumbers wide at the base, narrower than O-H stretching peaks that can be upwards of a thousand wavenumbers wide. However, these N-H stretches are broader than the C-H stretches seen in the spectrum just below 3000.
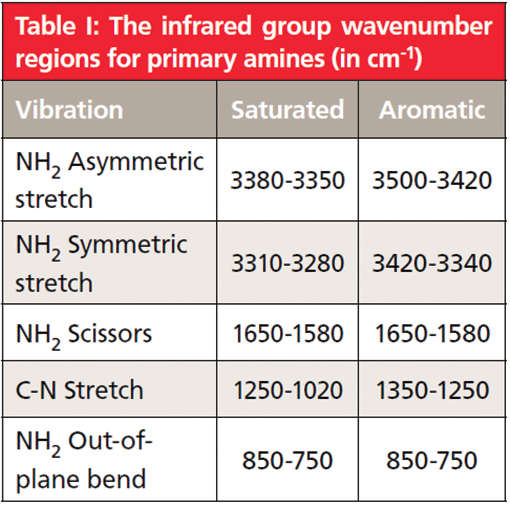
Table I: The infrared group wavenumber regions for primary amines (in cm-1)
As seen in Table I, saturated primary amine asymmetric stretching peaks fall from 3380 to 3350, whereas the saturated symmetric stretching peak appears between 3310 to 3280. Also note in Table I that there are separate N-H stretching peaks for saturated and aromatic primary amines. For primary aromatic amines, the asymmetric and symmetric N-H stretches fall from 3500 to 3420, and 3420 to 3340, respectively. Note that the N-H stretching peaks of saturated primary and aromatic primary amines are different from each other, which means these peaks can be used to distinguish primary saturated amines from primary aromatic amines. The pair of peaks labeled A and B in Figure 5 then carry an unusually large amount of information. The pair of N-H stretches means the amine is primary, and the peak positions mean the amine is saturated.
Quiz Section
The primary amine group also has a scissoring vibration, as seen in Figure 4. Both the water molecule and the methylene group have similar vibrations. According to Table I, the primary amine scissors peak falls from 1650 to 1580. Note that this peak position is the same whether the primary amine is saturated or aromatic. We have seen (4) that the water molecule's scissoring peak falls around 1630. However, the same maxim that applies to NH and OH stretches also applies to their bending vibrations; peaks from O-H bonds will generally be broader and more intense than those from N-H bonds. The propylamine N-H scissors peak at 1606 is labeled C in Figure 5. It shows up near where the water scissors peak appears, it is somewhat broadened, but is not wide enough to be mistaken for an OH bending peak.
The last useful group wavenumber for primary amines is the NH2 wagging peak. It falls from 850-750, as seen in Table I, and has the same range for both saturated and aromatic primary amines. In the spectrum of propylamine in Figure 5, it is found at 812 and is labeled E. Note that it is somewhat broadened like the primary amine N-H stretching and scissoring peaks, again due to hydrogen bonding.
A primary amine does contain a C-N bond, and, yes, there will be a C-N stretching peak as a result. However, as we discussed last time (1), because C-N stretches are not that polar, their peak intensities are not strong, and they have the misfortune of falling in the busy fingerprint region (1600 to 1000 cm-1) of the mid-infrared spectrum. The C-N stretch of propylamine is at 1072, and is labeled D in Figure 5. Note how small and nondescript this peak is. It can be seen here because propylamine is a simple molecule, with only a few peaks in its fingerprint region. For any molecule larger or more complex, this C-N stretching peak would be lost in the shuffle.
Conclusions
Amines are a class of nitrogen containing organic compounds that consist of C-H and C-N bonds. There are three types of amine: primary, secondary, and tertiary, which are determined by the number of C-N bonds in the functional group. As the number of C-N bonds in an amine group goes from one, to two, to three, the number of N-H bonds goes from two, to one, to zero. The nature of the carbons attached to the nitrogen also determines whether the amine is saturated or aromatic. There are six types of amines: primary saturated, primary aromatic, secondary saturated, secondary aromatic, tertiary saturated, and tertiary aromatic.
Primary amines contain an NH2 group, and their spectra are dominated by the stretching and bending of this moiety. Primary amines are distinguished from secondary amines by the presence of two N-H stretching peaks instead of one. The positions of these two peaks disclose whether the primary amine is saturated or aromatic. There are also two NH2 bending vibrations for primary amines that can be useful for identifying this functional group.
References
(1) Brian C. Smith, Spectroscopy, 34(1), 10–15 (2019).
(2) Brian C. Smith, Spectroscopy, 30(4), 18–23 (2015).
(3) A. Streitweiser and C. Heathcock, Introduction to Organic Chemistry (Macmillan, New York, 1976).
(4) Brian C. Smith, Spectroscopy, 31(1), 14–21 (2016).
Brian C. Smith, PhD, is founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer reviewed papers, and has written 3 books on spectroscopy. As a trainer, he has helped thousands of people around the world improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at: SpectroscopyEdit@UBM.com

The Big Review V: The C-O Bond
March 20th 2025In the fifth installment of “The Big Review” of infrared (IR) spectral interpretation, we review the spectroscopy of functional groups containing C-O bonds, discuss alcohols and phenols, and see how to use IR spectroscopy to distinguish these alcohols from each other. We then discuss ethers and see how to use IR spectroscopy to distinguish the three different types from each other.
The Big Review IV: Hydrocarbons
January 25th 2025In the fourth installment of our review of infrared spectral interpretation, we will discuss the spectroscopy of hydrocarbons. We will look at the stretching and bending vibrations of methyl (CH3) and methylene (CH2) groups, how to distinguish them, and how to know whether one or both of these functional groups are present in a sample. We will also discuss aromatic hydrocarbons, specifically the C-H stretching and bending peaks of mono- and disubstituted benzene rings, and how to distinguish them.
The Big Review III: Molecular Vibration Theory
January 2nd 2025It has occurred to me that, in the 10+ years I have been writing about molecular vibrations, I have never introduced my readers to its basic theory! I will rectify that now. Some of this is new material, and some will be review. Either way, it is important that all this material be covered in one place.
The Big Review II: The Physical Mechanism of Infrared Absorbance and Peak Types
October 10th 2024In the second installment of “The Big Review,” we discuss the physical mechanism behind how molecules absorb infrared (IR) radiation. Because light can be thought of as a wave or a particle, we have two equivalent pictures of IR absorbance. We also discuss the quantum mechanics behind IR absorbance, and how this leads to the different peak types observed in IR spectrum.
