Organic Nitrogen Compounds, VII: Amides—The Rest of the Story
Spectroscopy
The N-H stretching and bending peaks can be used to distinguish primary, secondary, and tertiary amides and to ascertain protein structure. Here’s how.
After introducing amides in the last column, in this installment we look at their infrared spectra. We will see how N-H stretching and bending peaks are used to distinguish primary, secondary, and tertiary amides from each other. We also briefly discuss how infrared spectroscopy can be used to ascertain protein structure.
Brian C. Smith
In our last installment, I introduced you to the amide functional group (1). Here, I tell the rest of their story-specifically, how to interpret their infrared spectra.
The generic molecular structure of amides is seen in Figure 1. As you may recall, amides are organized into three types: primary, secondary, and tertiary, as seen in Figure 2. The difference between them is the number of C-N bonds, with the three amide types having 1, 2, or 3 of these bonds, respectively. Additionally, the number of N-H bonds goes down as the number of C-N bonds goes up, with primary amides having an NH2 group, secondary amides having an N-H group, and tertiary amides having no N-H bonds.
Figure 1: The generic structural framework of the amide functional group.
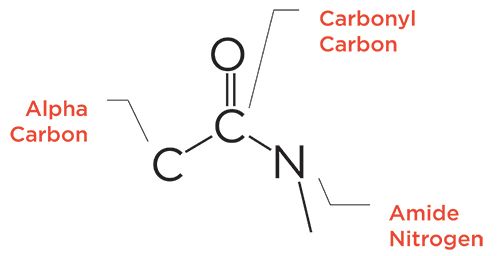
Figure 2: The skeletal frameworks of primary, secondary, and tertiary amides.
Similar to carbonyl groups attached to a benzene ring (2), amides undergo conjugation. As a result, their carbonyl stretch is lower than that of many other similar functional groups, and this peak falls for all amides from 1680 to 1630. Note, however, that we do not speak of “saturated” or “aromatic” amides, as conjugation takes place in all amides, so they all have the same carbonyl stretching peak range. Amides also engage in hydrogen bonding. However, like amines, the strength of the hydrogen bonding is less than that for O-H bonds, giving medium width and intensity N-H stretching peaks compared to O-H stretches.
Primary Amides
The infrared spectrum of a primary amide, benzamide, is seen in Figure 3.
Figure 3: The infrared spectrum of benzamide (C7
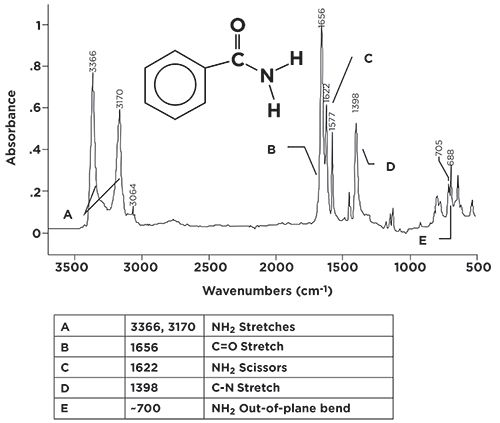
H7NO), a primary amide.
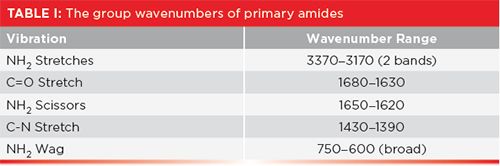
Because this molecule has an NH2 group, it has two N-H stretching vibrations, whose peaks are seen at 3366 and 3710 (going forward, assume all peaks positions stated are in cm-1 units, even if not stated). Note that these peaks fall in the same range as O-H stretches, but you should never confuse the two, because N-H stretching peaks are narrower, and less intense, than O-H stretching peaks. Note that these two peaks come to a nice sharp point, but their bases are broadened due to hydrogen bonding. At their base, these two peaks are about 400 cm-1 wide, whereas O-H stretching envelopes are often more than 1000 cm-1 wide. For primary amides in general, this pair of peaks falls between 3370 and 3170 cm-1 Again, both peaks must be present for a molecule to be a primary amide. The C=O stretch in Figure 3 is seen at 1656, well within the range expected for amides.
Primary amides have a structural similarity to primary amines in that they both contain the NH2 group. Recall that primary amines have scissoring and wagging bending vibrations (3), and so do primary amides. In Figure 3, the primary amide scissoring peak is seen at 1622, and, in general, this peak falls from 1650 to 1620. The NH2 wag is a broadened envelope, due to hydrogen bonding, around 700. This envelope normally tops out between 750 and 600. The pair of sharp peaks on top of this envelope are the C-H wag and ring bend of a mono-substituted benzene ring (4), which is present in benzamide.
In a previous column (3), I pointed out that C-N stretches are not useful group wavenumbers, since they are usually medium-to-weak in intensity, and fall from 1400 to 1000, right in the middle of the very busy fingerprint region, where it is easy for them to get lost. The C-N stretch of benzamide is seen at 1398. This particular molecule has a relatively simple spectrum, so this peak is visible, but you can see how it might get lost if there were other peaks of similar size around it. A summary of the group wavenumbers for primary amides is seen in Table I.
Secondary Amidesare NOT Secondary
The secondary amide linkage is important, and is found all around and within us. A number of polymeric materials, including the nylon family, contain secondary amide linkages. Also, the amino acid units in proteins are held together by secondary amide linkages. The infrared spectrum of a secondary amide, Nylon-6,6 is seen in Figure 4.
Figure 4: The infrared spectrum of the polymeric secondary amide Nylon-6,6 (repeat unit C12H22N2O2).
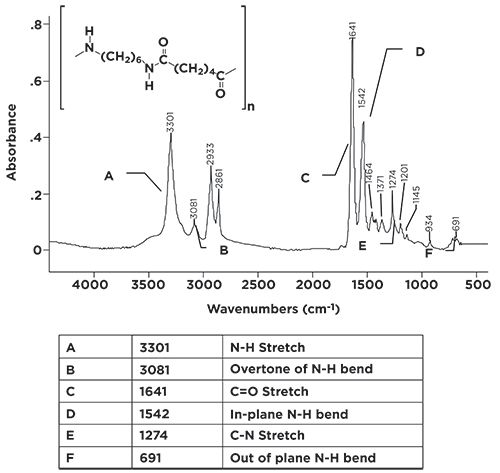
In nylon nomenclature, the two numbers refer to the number of carbons between the nitrogens. For Nylon-6,6, there are six carbons between the nitrogens, for Nylon-4,4, there would be four, and so on.
Because secondary amides contain one N-H bond, there is only one N-H stretching peak, seen at 3301 in Figure 4. The easiest way then to distinguish a primary amide from a secondary amide is to remember that, because a primary amide has a NH2 group, it has two N-H stretching peaks, whereas a secondary amide has one N-H bond, and hence one N-H stretching peak. Note the peak at 3301 in Figure 4 is of medium intensity and width, like all the other N-H stretching peaks we have seen. For secondary amides, this peak generally falls from 3370 to 3170.
The C=O stretch of our secondary amide is seen in Figure 4 at 1641, in the 1680 to 1630 range typical of all amides. Note, however, it has a companion peak at 1542. This is the in-plane N-H bend of the secondary amide group, and is normally found from 1570 to 1515. This peak’s position is unusual in that there are very few group wavenumber peaks that fall around 1550, and it is unusually intense for an in-plane bend. We have said very little about in-plane bends throughout this column series, because they are generally weak and typically fall in the middle of the busy fingerprint region where they are easily lost. This in-plane bend is higher in wavenumber and more intense than others because of the conjugation that takes place in amides (5). The C=O and N-H in plane bend peaks form a diagnostic pair of sharp, intense peaks in the middle of the spectrum. Combine these with the single N-H stretch of secondary amides and you have a trio of peaks that clearly indicate when a secondary amide is present in a sample. For the nylon family of polymers particularly, this pair peaks falls at ~1640 and ~1540, and are easily spotted. The N-H wag of secondary amides forms a broadened envelope from 750 to 680 and is seen in Figure 4 at 691. The summary of secondary amide group wavenumbers is seen in Table II.
Proteins
Proteins are polymers that consist of amino acid repeat units. The repeat units are linked together by secondary amide linkages. The spectrum of a protein, keratin, is seen in Figure 5.
Figure 5: The infrared spectrum of the protein keratin (wool fibers).
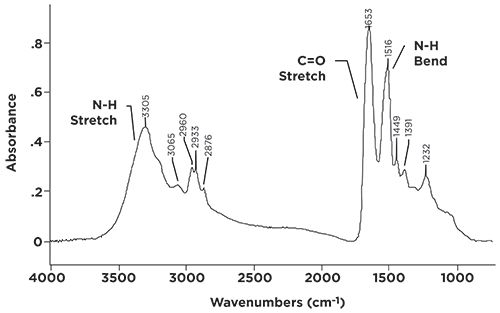
Mammal hair, skin, feathers, and reptile scales are all made from keratin, so it is a commonly found material. The spectrum in Figure 5 is of sheep hair (wool). Note that the spectrum is dominated by secondary amide peaks, with N-H stretch, C=O stretch, and N-H in-plane bend peaks clearly visible.
I am often asked by my students in the infrared spectral interpretation training courses I teach whether infrared spectroscopy can be used to distinguish between natural and synthetic materials. I am afraid that, in general, the answer is no. Remember that infrared spectroscopy is sensitive to the functional groups present in a sample. Functional groups generally have the same structure, whether they are made in a test tube or a living cell. Figure 5 shows this; if I didn’t know this spectrum were of a protein, I might have thought it was a synthetic secondary amide, such as a nylon.
What about tertiary amides? As seen in Figure 1, this functional group has no N-H bonds, hence no N-H stretching and bending peaks, and hence no useful group wavenumbers. Tertiary amides will exhibit a C=O stretch from 1680 to 1630 like all other amides, but there are many conjugated C=O functional groups whose carbonyl peaks also fall in this range, meaning there is nothing unique about the spectra of tertiary amides. It is an example of a functional group that are not readily detected by infrared spectroscopy.
Conclusions
Amides come in primary, secondary, and tertiary forms. The diagnostic pattern of peaks for primary amides is a pair of N-H stretching peaks and a C=O stretch. The pattern for secondary amides is one N-H stretch, a C=O stretch, and an intense N-H in-plane bend next to the C=O stretch. Proteins are an important class of molecules whose spectra are dominated by secondary amide peaks. Unfortunately, there is not a good way of using infrared spectroscopy to distinguish natural from synthetic secondary amides. Tertiary amides are difficult to detect via infrared spectroscopy.
References
- B.C. Smith, Spectroscopy34(11), 30–33 (2019).
- B.C. Smith, Spectroscopy32(9), 31–36 (2017).
- B.C. Smith, Spectroscopy 34(3), 22–25 (2019).
- B.C. Smith, Spectroscopy 31(7), 30–34 (2016).
- B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, Florida, 1999).
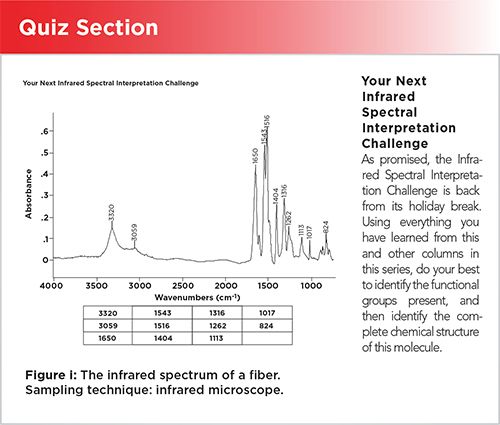
Your Next Infrared Spectral Interpretation Challenge
As promised, the Infrared Spectral Interpretation Challenge is back from its holiday break. Using everything you have learned from this and other columns in this series, do your best to identify the functional groups present, and then identify the complete chemical structure of this molecule.

Brian C. Smith, PhD, is the founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer reviewed papers, and has written three books on spectroscopy. As a trainer, he has helped thousands of people around the world improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at: SpectroscopyEdit@MMHGroup.com

Artificial Intelligence Accelerates Molecular Vibration Analysis, Study Finds
July 1st 2025A new review led by researchers from MIT and Oak Ridge National Laboratory outlines how artificial intelligence (AI) is transforming the study of molecular vibrations and phonons, making spectroscopic analysis faster, more accurate, and more accessible.
Toward a Generalizable Model of Diffuse Reflectance in Particulate Systems
June 30th 2025This tutorial examines the modeling of diffuse reflectance (DR) in complex particulate samples, such as powders and granular solids. Traditional theoretical frameworks like empirical absorbance, Kubelka-Munk, radiative transfer theory (RTT), and the Hapke model are presented in standard and matrix notation where applicable. Their advantages and limitations are highlighted, particularly for heterogeneous particle size distributions and real-world variations in the optical properties of particulate samples. Hybrid and emerging computational strategies, including Monte Carlo methods, full-wave numerical solvers, and machine learning (ML) models, are evaluated for their potential to produce more generalizable prediction models.
Polystyrene and UVC Sterilization Tested with Spectroscopy and Luminescence Tools
June 25th 2025A team of researchers from Spanish institutions has found that polystyrene used in healthcare packaging shows strong resistance to UVC sterilization, with minimal chemical degradation detected using FT-IR and Raman spectroscopy.