Probing Aqueous Surfaces by TOF-SIMS
Special Issues
A breakthrough using a microfluidic interface to conduct sensitive time-of-flight secondary ion mass spectrometry (TOF-SIMS) analysis and study liquid surfaces in situ under vacuum conditions is described here.
We report the first observations of aqueous surfaces by time-of-flight secondary ion mass spectrometry (TOF-SIMS) via a self-contained microfluidic module compatible with a vacuum. The interface uses a microfluidic channel with a 3-µm diameter window into the flowing fluid beneath it. This window supports the liquid against the vacuum by the liquid's surface tension and limits the high-density vapor region traversed by the probe beams to only a few micrometers. We demonstrate detection of aqueous surfaces such as deuterium water and sodium iodide (NaI) solution through the small aperture by TOF-SIMS. A molecular signal (C5H8NO4– = [M-H]-) of glutamic acid also was observed. TOF-SIMS coupled with the interface provides a molecular recognition capability, making it a great choice to detect short-lifetime reaction intermediates in aqueous solutions. This novel microfluidic interface makes multimodal vacuum-based analysis of liquid surfaces possible.
This article describes a breakthrough using a microfluidic interface to conduct sensitive time-of-flight secondary ion mass spectrometry (TOF-SIMS) analysis and study liquid surfaces in situ under vacuum conditions. Applications in TOF-SIMS so far have been limited to solid surfaces or low-vapor-pressure liquid surfaces.
TOF-SIMS has been applied in applications such as polymers (1), pharmaceuticals (2), semiconductors (3), environmental chemistry (4), inorganic surfaces (5), corrosion and monitoring of organic coating processes, catalysis (6), investigation of surface contamination (7), geology (8), and archaeology (9). However, a lot of chemistry takes place at the interface of liquid phases with gases in environmental, industrial, and biological systems. The surfaces of these aqueous phases and films (when <1-nm thick) have unique kinetics and thermodynamics that are distinct from those of the bulk materials (10–12). Although TOF-SIMS has already been used to investigate ionic liquids (13), lubricants (14,15), and liquid crystals (16,17), which all have very low vapor pressure, no one has been able to analyze high-vapor-pressure aqueous solutions (to the best of our knowledge). Because TOF-SIMS is vacuum-based, one cannot easily probe these high-vapor-pressure interfaces. Our goal is to permit studies of high-vapor-pressure liquid surfaces in TOF-SIMS.
TOF-SIMS offers great sensitivity and attractive spatial resolution as an advanced vacuum-based surface analytical technique. It uses a focused ion beam to raster over the surface, ejecting molecules, atoms, and molecular fragments (neutral and ionic) from the top layer, and a TOF mass spectrum is obtained for each pixel (see Figure 1). TOF-SIMS gives high mass resolution (m/Δm > 10,000) and high lateral resolution (<100 nm), a great advantage in exploring complex samples. Automated charge compensation permits the study of insulators. Simultaneous auxiliary ion sputtering permits depth profiling and 3D chemical maps. Negative or positive emitted ions can be monitored. Sensitivity is typically in a range of 0.1–10 ppm.
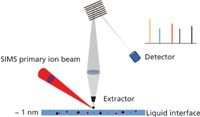
Figure 1: A schematic of the TOF-SIMS instrument.
In this article, we will describe the first aqueous surface detections by TOF-SIMS enabled by a microfluidic interface designed for operation in vacuum conditions. We demonstrate new TOF-SIMS applications using different liquid samples prepared in the laboratory. Directions for using this unique technique in future developments are also presented.
Methods and Materials
Device Fabrication
A mixture of polydimethylsiloxane (PDMS) prepolymer and curing agent (Sylgard 184, Dow Corning Co., Midland, Michigan) with a 10:1 ratio (w/w) was used to make the microfluidic device via a soft photolithography technique (18). The microchannel was 80-µm wide and 2.97-mm long, and the narrow part of the channel in the middle was 10-µm wide and 30-µm long. The depth of the whole channel was 8 µm. The flow channel was enclosed using a silicon nitride (SiN) window that contained either a 100-nm-thick SiN membrane (0.5 mm × 0.5 mm) and a frame with 200-µm thick silicon (7.5 mm × 7.5 mm) or a 500-nm-thick SiN membrane (1.5 mm × 1.5 mm) and a frame with 200-µm thick silicon (7.5 mm × 7.5 mm) (Norcada Inc., Canada) by oxidizing the membrane and frame in an oxygen plasma (PX-250; March Plasma Systems, Concord, California) for 1 min and immediately bringing them into conformal contact (18) under a stereomicroscope (SMZ-U, Nikon, Japan). The assembly was then placed in an oven at 75 °C for 2 h to form an irreversible bond. Above the channel on the SiN window, a hole approximately 2.5 µm in diameter was drilled through the membrane by using the TOF-SIMS depth profiling feature (see Figure 2).
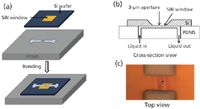
Figure 2: Fabrication of the vacuum-compatible microfluidic interface. (a) A schematic showing the assembly of the microfluidic block; (b) a cross-section view of the PDMS block showing the aperture; and (c) an optical image showing the top view of the SiN window with the aperture as a part of a finished device.
Interface Assembly
The aqueous vacuum interface module is shown in Figure 3. It is composed of a PDMS microfluidic block; an electro-osmotic pump (model number 3000126, Dolomite, United Kingdom); a battery (Saft, Li-SOCl2); and PTFE connecting tubing (Valco Instruments Co. Inc., Houston, Texas). The PDMS microfluidic block was coated with a thin layer of gold film, except for the membrane window. The continuous flow in the channel was obtained by an electro-osmotic pump that was driven by a battery. The wire connections were covered by ultrahigh vacuum (UHV)-compatible fiberglass sleeving. A Viton tube was intersected with a short capillary (360 µm o.d. and 74 µm i.d., Polymicro Technologies, Phoenix, Arizona) to form a tee. Various aqueous solutions were introduced into the system using a syringe pump (Harvard Apparatus, Holliston, Massachusetts).

Figure 3: A photo of the interface including: (a) PMDS block containing the microchannel, (b) electro-osmotic pump, (c) battery, (d) one of two PTFE tube fluid reservoirs, ( e) connecting wires to battery, and (f) pressure relief tee (see text).
Battery Adaptation
A commercial electro-osmotic pump was adapted for the dynamic microchip design. By applying a voltage gradient along the water flow path inside the pump, the liquid is moved in uniform "plug flow" and is able to run on low voltages. For our flow and channel back pressure, it requires only about 3 V bias. Different solutions were used. Because the pumping mechanism requires deionized (DI) water, the liquid of interest is placed past the DI water and separated by a small section of inert perfluorodecalin liquid as an immiscible spacer.
This pump is designed to be used with open-to-air reservoirs connected to microfluidic systems; thus, it is not well configured to be inserted directly in-line on our microfluidic device. Problems such as electrodes springing fluid leaks in vacuum have been encountered. To solve these problems, two aluminum blocks with O-rings were used to seal the pump between them.
Instrumentation
A TOF-SIMS V spectrometer (IONTOF GmbH, Münster, Germany) was used to study the aqueous interface in this work. A pulsed 25-keV Bi+ (beam size: ~250 nm) ion beam with an incident angle of 45° off the normal was used as the primary ion beam for all measurements. The main chamber operating vacuum pressure during measurements was 2.5–5.5 × 10-7 mbar. The interface device is normally preconditioned in a vacuum chamber for leak checks before usage. The instrument can be easily run on Bi cluster ions (for example, Bi3+ or Bi32+), which may give higher sensitivity for molecular ions. The SIMS measurements were performed at the beam current of 1.0 pA with a beam width of 130 ns and chopped at 20 kHz. Images shown in this article were taken for a total integration time of 65.5 s, over a scan area of 10 µm × 10 µm, with 256 × 256 pixels.
Solution
Various liquids, including DI water, deuterium water (Sigma-Aldrich Corp., St. Louis, Missouri, 99.9%), sodium iodide (Sigma-Aldrich Corp., 99.99%), and 0.5% glutamic acid aqueous solutions, were prepared in the laboratory and filled in the system by a syringe pump.
Results and Discussion
This is the first time, to the best of our knowledge, that highly volatile liquid surfaces have been investigated using TOF-SIMS. The TOF-SIMS primary ion beam is used to drill the aperture by turning up the ion beam current. In-situ drilling reduces the handling of the whole device before usage and possible contamination, compared to making the hole in advance with other techniques. In this experiment, the channel contained flowing D2O. The focused 250 nm Bi+ beam was rastered over a circular area with a diameter of 3 µm. To get a rapid sputtering rate, a high average current was required, and it was achieved by lengthening the pulse width to 1000 ns. High mass resolution spectra (narrow pulse width) show that the H2- peak is very weak (<1% of D-), and thus the signal around the 2.0 amu range can be regarded as pure D-. Signals of H-, D-, and O- were monitored by TOF-SIMS yielding a depth profile, while the intense sputtering was ongoing. Figure 4 shows that the Bi+ beam can drill a hole through the 500-nm SiN layer in about 175 s. The D- signal shows a dramatic jump (~300-fold in signal amplitude) as soon as the Bi+ beam passes through the SiN membrane. Signals of H- and O- only show a small jump (eightfold for H- and 10-fold for O-) compared with D-. At the initial punch-through, probably only a small and irregular hole is formed and the size of the hole becomes larger and larger with additional doses of Bi+ ions, until finally the hole becomes about 3 µm in size and the D- and H- signals become constant. The reason why the signal spikes higher for a few seconds initially is most likely due to pressure in the channel. The inset image in Figure 4 shows an optical image of a TOF-SIMS-drilled hole after Bi+ bombardment.
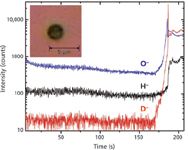
Figure 4: O-, H-, and D-signals vs. Bi+ion erosion time, while a hole into the channel is being drilled by TOF-SIMS. An optical image (10 µm à 10 µm) of the hole created is shown in the insert.
Figures 5a and 6a show the positive and negative secondary ion images, respectively, around the aperture with the channel left unfilled. In these TOF-SIMS images, the lighter yellow color indicates high intensity of the element being detected and the darker color indicates low intensity of the element. A clear hole can be found at the center of the Si+ image with a diameter about ~2 µm, which is consistent with our design, indicating that there is no existence of Si in the aperture (19). In addition, very low Na+ (Figure 5a), H-, and I- (Figure 6a) signals are observed in the hole.

Figure 5: Left: Positive TOF-SIMS images (10 µm à 10 µm) of an SiN membrane surface around the detection aperture: The microchannel was (a) empty, (b) filled with 5 mM NaI aqueous solution, (c) filled with deuterium water, or (d) filled with 0.5% glutamic acid solution. Right: Positive TOF-SIMS spectra of 5 mM NaI aqueous solution in the hole and out of the hole.
Figures 5b and 6b show the case where a 5 mM NaI aqueous solution is flowing through the channel using the same conditions as those of Figure 5a. The Si+ image shows a hole, as expected. The Na+ signal has a very bright core, about the same size as the Si+ hole. The corresponding positive spectra collected from the hole show significant sodium and silicon signals at m/z 23 and 28, which are consistent with the positive images. However, when comparing positive spectra obtained from out of the hole, there is reasonable Si+ signal but no signal for Na+. This indicates that the aqueous surface containing sodium ions can be probed in the hole. Figure 6b shows that the I- signal originates from a region about the size of the Si+ hole. This is compatible with what is expected for imaging the liquid surface. The H- signal is intense at the region where water is expected. Our results show that the aqueous solution is exposed to the vacuum, and its composition can be probed by the TOF-SIMS.

Figure 6: Left: Negative TOF-SIMS images (10 µm à 10 µm) of an SiN membrane surface around the detection aperture: The microchannel was (a) empty, (b) filled with 5 mM NaI aqueous solution, (c) filled with deuterium water, or (d) filled with 0.5% glutamic acid solution. Right: Negative TOF-SIMS spectra of deuterium water in and out of the hole.
Figures 5c and 6c show the case where deuterium water was filled in the channel. The Si+ image shows a hole and the Na+ signal is scarcely observed, both of which were as expected. Strong D- and OD- signals were seen, which also was expected from the deuterium water. The corresponding negative spectra collected from the hole show significant D- and OD- signals at m/z 2 and 18 compared with those obtained from out of the hole, which are consistent with the negative ion images.
An important advantage of TOF-SIMS is that it can provide molecular signals. When a 0.5% (weight ratio) glutamic acid (C5H9NO4) aqueous solution was flown through the channel, a [M-H]- molecular signal was clearly observed in the liquid exposed at the hole, compared to the area outside the aperture (see Figure 6d). Understanding what organic or biological molecules are exposed to at the surface of a solution could be important in many applications. This interface makes it possible to explore those systems.
Conclusions
We report the application of TOF-SIMS in probing liquid surfaces by using a novel portable microfluidic device, which enabled TOF-SIMS to study room-temperature aqueous surfaces for the first time. The modular design of the microfluidic interface makes it potentially adaptable to many other vacuum-based analytical techniques. That is, multimodal analysis of the same sample will be made possible by our portable platform. Since the interface is based on microfluidics, hyphenated analytical techniques using liquid chromatography (LC) or capillary electrophoresis (CE) on a microchip with TOF-SIMS are possible. This has added value in the multidimensional analytical technique because it will allow us to study selected liquid surfaces after separation and decipher reactions occurring in complicated mixtures and matrices. To sum up, this novel development will open new avenues for understanding interfacial reactions occurring on aqueous surfaces in biological and environmental studies in the future.
Acknowledgments
Support from the Department of Energy (DOE) Division of Chemical Sciences, Geosciences, and Biosciences (BES Chemical Sciences grant, KC-0301020-16248) is gratefully acknowledged. The research was performed in the W.R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the DOE's Office of Biological and Environmental Research (OBER) and located at the Pacific Northwest National Laboratory (PNNL). PNNL is operated for DOE by Battelle. A Battelle patent (Intellectual Property Report No. 16961-E) was filed based on this invention.
Xiao-Ying Yu is a senior research scientist in the Atmospheric Sciences and Global Climate Change division at Pacific Northwest National Laboratory in Richland, Washington. Direct correspondence to: xiaoying.yu@pnnl.gov.
Li Yang is a postdoctoral research associate in the Chemical and Materials Sciences division at Pacific Northwest National Laboratory in Richland, Washington.
Zihua Zhu is a senior research scientist in the Scientific Resources Division at W. R. Wiley Environmental Molecular Science Laboratory, Pacific Northwest National Laboratory, Richland, Washington.
James P. Cowin was formerly a chief scientist in the Chemical and Materials Sciences division at the Pacific Northwest National Laboratory in Richland, Washington. He is now the President of Cowin In-Situ Science, LLC in Richland, Washington.
Martin J. Iedema is a senior research scientist in the Scientific Resources Division at W. R. Wiley Environmental Molecular Science Laboratory, Pacific Northwest National Laboratory in Richland, Washington.
References
(1) Y.-T.R. Lau, L.-T. Weng, and K.-M. Ng, Surf.and Inter. Anal. 43, 340–343 (2011).
(2) E.A. Jones, N.P. Lockyer, and J.C. Vickerman, Int. J. Mass Spectrom. 260, 146–157 (2007).
(3) H. Masudome and H. Abe, Surf. Interface Anal. 43, 664–668 (2011).
(4) D.J. Gaspar, A. Laskin, and W. Wang, Appl. Surf. Sci. 231, 520–523 (2004).
(5) J.A. Denman, W.M. Skinner, and K.P. Kirkbride, Appl. Surf. Sci. 256, 2155–2163 (2010).
(6) J. Grams, J. Goralski, and T. Paryjczak, Surf. Sci. 549, L21–L26 (2004).
(7) I.A. Mowat, T. Schuerlein, and J. Metz, in 6th International Symposium on Cleaning Technology in Semiconductor Device Manufacturing, R.E. Novak, J. Ruzyllo, and T. Hattori, Eds. (Honolulu, Hawaii, Vol. 99, 2000) pp. 561–568.
(8) A.F.A. Marques, S.D. Scott, and R.N.S. Sodhi, Surf.and Inter. Anal. 43, 436–442 (2011).
(9) R.N.S. Sodhi, W.C. Mahaney, and M.W. Milner, Appl. Surf. Sci. 252, 7140–7143 (2006).
(10) N. Altimir, P. Kolari, J.-P. Tuovinen, T. Vesala, J. Back, T. Suni, M. Kulmala, and P. Hari, Biogeosci. 3, 209–228 (2006).
(11) A.L. Sumner, E.J. Menke, Y. Dubowski, J.T. Newberg, R.M. Penner, J.C. Hemminger, L.M. Wingen, T. Brauers, and B.J. Finlayson-Pitts, Phys. Chem. Chem. Phys. 6, 604–613 (2004).
(12) S.C. Ringwald and J.E. Pemberton, Environ. Sci. Technol. 34, 259–265 (2000).
(13) M. Holzweber, E. Pittenauer, and H. Hutter, J. Mass Spectrom. 45, 1104–1110 (2010).
(14) R. Ji, T. Liew, and T.C. Chong, Appl. Surf. Sci. 255, 1490–1493 (2008).
(15) R. Ji, J. Xu, T. Liew, J. Zhang, H.Q. Xie, B. Xu, and T.K. L. Dao, SIMS Proceedings Papers 43, 406–409 (2011).
(16) J. Grams and I. Sobczak, Int. J. Mass Spectrom. 289, 138–143 (2010).
(17) Y.-T. R. Lau, L.-T. Weng, K.-M. Ng, and C.-M. Chan, Surf. Inter. Anal. 42, 1445–1451 (2010).
(18) J.C. McDonald and G.M. Whitesides, Acc. Chem. Res. 35, 491–499 (2002).
(19) L. Yang, X.-Y. Yu, Z. Zhu, M.J. Iedema, and J.P. Cowin, Lab Chip 11, 2481–2484 (2011).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.