Rapid Analysis of Selected Benzodiazepines by Automated SPE–MS-MS
Special Issues
A new method was developed and validated using automated on-line solid-phase extraction (SPE) with tandem mass spectrometry (MS). Urine samples were enzyme-hydrolyzed and diluted before detection. The validated method was applied to positive authentic urine samples to evaluate concordance with high performance liquid chromatography (HPLC)–MS-MS results.
A new method was developed and validated using automated on-line solid-phase extraction (SPE) with tandem mass spectrometry (MS). Urine samples were enzyme-hydrolyzed and diluted before detection. The validated method was applied to positive authentic urine samples to evaluate concordance with high performance liquid chromatography (HPLC)–MS-MS results.
Benzodiazepines were first synthesized in the 1950s and successfully marketed in 1960 (1,2), leading to a rapid rise in prescriptions of the compounds for the treatment of anxiety, insomnia, and seizures, among other conditions (3). They were seen as safer than, but just as effective as, barbiturates and other older drugs used as sedatives and hypnotics. However, by the 1980s it became clear that benzodiazepines were also saddled with the same liabilities for abuse and dependence as the older medications they replaced (1,4). Despite this risk, as well as the availability of other safer medications with anxiolytic properties, the number of prescriptions written for benzodiazepines has not significantly dropped (1,4). In 2012, three benzodiazepines (alprazolam, lorazepam, and clonazepam) were among the top 100 drugs prescribed in the United States, while two others (diazepam and temazepam) made it onto the top 200 list (5). Because benzodiazepines are so widely available, both by prescription and illicitly, and have the potential to be abused, there is a need for patients to be monitored for therapeutic drug compliance or diversion.
The class of drugs termed benzodiazepines share the core structural components of a benzene ring fused with a diazepine ring (3). The unique side chains found in distinct benzodiazepine compounds give each drug its pharmacological properties, modulating its binding to the GABAA receptor (2,4). Benzodiazepines derive their pharmacological actions from their interactions with the GABAA receptor, increasing the efficiency of GABA, thereby reducing communication between neurons and yielding a calming effect on many brain functions (6). In addition to these analgesic effects, benzodiazepines are often misused by poly-drug abusers to enhance and prolong the "high" generated by other drugs, and ease the withdrawal effects when other drugs are scarce (2,6,7). These unintended uses have resulted in widespread abuse of benzodiazepines and have led to their regulation by the U.S. Drug Enforcement Agency (DEA) as Schedule IV drugs (2), thereby supporting the need for urine drug testing for compliance and abuse of these compounds.
There are more than 50 benzodiazepine compounds marketed worldwide (4), but the focus of this work is on seven of the more commonly prescribed compounds or their major metabolites: 7-aminoclonazepam, α-hydroxyalprazolam, alprazolam, lorazepam, nordiazepam, oxazepam, and temazepam (structures depicted in Figure 1). In this study, quantitative results for the analysis of these seven benzodiazepines and metabolites using a solid-phase extraction tandem mass spectrometry (SPE–MS-MS) method are compared with earlier results obtained using a conventional high performance liquid chromatography (HPLC)–MS-MS method. Because these drugs undergo extensive metabolism, samples are hydrolyzed to return compounds to their nonglucuronidated state before confirmation analysis. The previous HPLC–MS-MS method used to analyze these drugs required a 6.4-min cycle time with a 96-s data window, such that quadruple multiplexing yielded a net time per sample of 96 s. The analytical columns for this method were replaced every 1000 injections of running in a production environment, while the guard columns were replaced every 1500 injections. The SPE-MS-MS method reported herein has been tested extensively, affording a comparison with the previous method for analysis time, cartridge lifetimes, data values, and overall cost per sample.
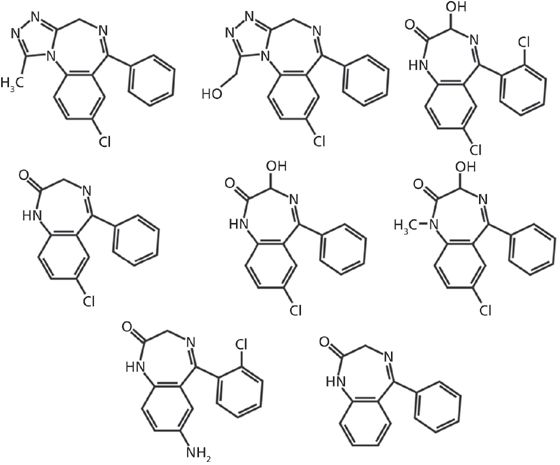
Figure 1: Chemical structures for the seven benzodiazepines analyzed. Row 1 (top), left to right: alprazolam, α-hydroxyalprazolam, lorazepam. Row 2 (middle), left to right: nordiazepam, oxazepam, temazepam. Row 3 (bottom), left to right: 7-aminoclonazepam, generic benzodiazepine backbone.
Experimental
Materials
The compounds of interest and the internal standard (alprazolam, α-hydroxyalprazolam, 7-aminoclonazepam, lorazepam, nordiazepam, oxazepam, temazepam, and nordiazepam-D5), along with the hydrolysis control (oxazepam glucuronide), were purchased from Cerilliant. Acetone (Optima grade), isopropanol (Optima grade), glacial acetic acid (certified ACS grade), formic acid (88%, laboratory grade), ammonium acetate (HPLC grade), ammonium formate (certified grade), and ammonium hydroxide (certified ACS Plus grade) were obtained from Fisher Scientific. Acetonitrile (OmniSolv gradient grade) and methanol (OmniSolv gradient grade) were acquired from EMD Chemicals. Potassium hydroxide (50% w/v, analytical reagent grade) was purchased from Ricca Chemical Company. Normal human urine was acquired from UTAK Laboratories. β-Glucuronidase (100,000 units/mL, from abalone) was purchased from Campbell Science. Water used for all reagents, mobile phases, and solvents was obtained from a Barnstead Nanopure analytical laboratory water system (18.2 MΩ·cm). The internal standard compound was diluted to a concentration of 1 μg/mL with water, while all other reference standards were diluted to concentrations of 100 and 10 μg/mL with methanol. These stock solutions were then used to prepare standard curve solutions and controls by diluting known amounts with normal human urine.
Methods
A stock enzyme buffer was prepared by combining 800 mL of water and 111 mL of glacial acetic acid and filling a 1-L flask to volume with the potassium hydroxide solution. This buffer was stored in the refrigerator and was stable for one month. The stock buffer was further diluted 1:10 in water for use in samples. The purchased β-glucuronidase was also diluted 1:5 in water for use in samples. To 0.5-mL aliquots of patient urine samples, 100 ng of internal standard was added, followed by 100 μL of diluted enzyme buffer (pH 4.7), and 20 μL of diluted enzyme. Samples were mixed by vortex and hydrolyzed at 60 °C for 30 min, then centrifuged for 10 min at 2500 rpm. For HPLC–MS-MS analysis, 96-well sample plates were placed directly in the autosampler. For on-line SPE-MS-MS analysis, samples were further diluted 20-fold in water before being loaded into the sample queue.
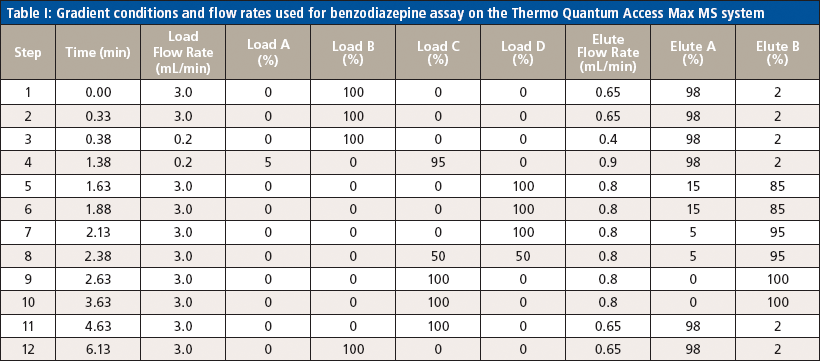
Table I: Gradient conditions and flow rates used for benzodiazepine assay on the Thermo Quantum Access Max MS system
Instrumentation
Liquid chromatography was conducted on an Agilent 1200 series HPLC system equipped with a 50 mm × 3 mm, 5-μm Thermo Hypersil GOLD aQ column fitted with a 50 mm × 0.5 mm Thermo Cyclone-P TurboFlow guard column. The injection volume was 10 μL with a total run time of 6.4 min and an injection-to-injection MS detection cycle time of 96 s. The flow rates and gradient conditions are detailed in Table I. Tandem MS was conducted using a Thermo TSQ Quantum Access Max MS system with electrospray ionization performed in the positive mode. The source temperature was 270 °C and the desolvation temperature was 375 °C. The compounds were analyzed in selected reaction monitoring (SRM) mode with argon as the collision gas. One quantitative transition and one qualitative transition were monitored for each analyte and internal standard. A summary of the MS parameters for each compound of interest are detailed in Tables II and III.
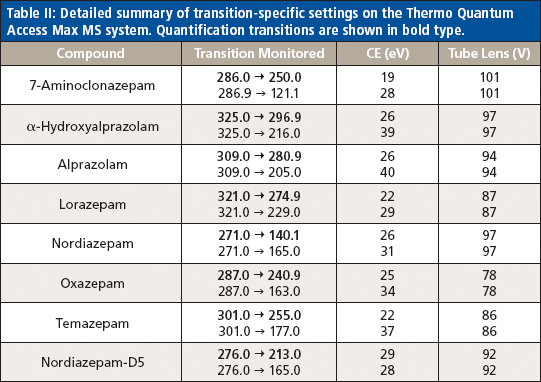
Table II: Detailed summary of transition-specific settings on the Thermo Quantum Access Max MS system. Quantification transitions are shown in bold type.
On-line SPE was conducted on an Agilent RapidFire 300 on-line SPE system. The SPE system uses a series of valves, high flow rate solvents, and proprietary cartridges to load a sample, rinse away the matrix, and then bulk elute or inject into a mass spectrometer in approximately 20 s or less. This method performs no chromatographic separation and therefore cannot resolve isobaric species. In this experiment, the injection volume was 10 μL onto an A2 C4 packed cartridge, with a total cycle time of 13 s. The on-line SPE cleanup of each sample was achieved with a sample load in water (mobile-phase A) at a flow rate of 1.5 mL/min and a wash of 10% acetonitrile in water (mobile-phase B) at a flow rate of 1.25 mL/min. The sample was eluted from the cartridge using 25:75 methanol–isopropanol (mobile-phase C) at a flow rate of 0.75 mL/min. Tandem MS was performed using either an Agilent 6460 triple-quadrupole system or an Agilent 6550 quadrupole time-of-flight (QTOF) system. Electrospray ionization was conducted in the positive mode. The gas temperature was 200 °C and the sheath gas temperature was 400 °C. The gas flow and sheath gas flow were set to 5 L/min and 11 L/min, respectively. The analytes of interest were detected using multiple reaction monitoring (MRM) mode with nitrogen as the collision gas. Two transitions, one quantitative and one qualitative, were monitored for each compound. A summary of the MS settings for each analyte are shown in Tables II and III. Quantitation by the SPE–MS-MS method was completed by correlating the ratio of the area counts of the analyte of interest to the area counts of the nordiazepam internal standard to the concentration of standards at varying concentrations using a quadratic curve and 1/x data weighting for each compound. The HPLC–MS-MS method was quantitated in a similar way, though there were matching deuterated internal standards for each analyte.
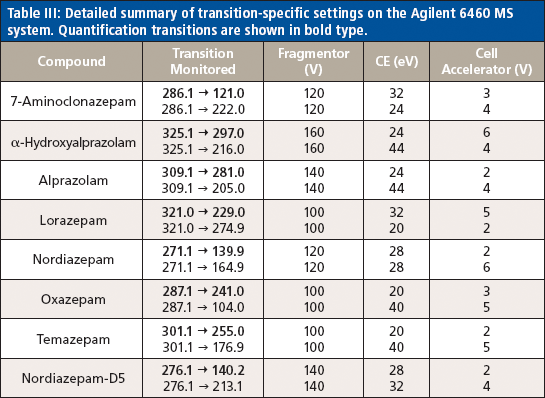
Table III: Detailed summary of transition-specific settings on the Agilent 6460 MS system. Quantification transitions are shown in bold type.
Results
All analytes displayed unique mass transitions, allowing the method to be easily converted to the automated SPE format, where no chromatographic separation occurs. A summary of the validation results from both the HPLC and the automated SPE methods are presented in Tables IV and V. To transition to the automated SPE method, a full validation procedure recommended by the College of American Pathologists (CAP) was performed, which included determination of the limit of detection (LOD), lower limit of quantitation (LLOQ), upper limit of quantitation (ULOQ), and an examination of carryover, matrix effects, interference, accuracy, intra- and inter-run precision, and patient comparison to the production method.
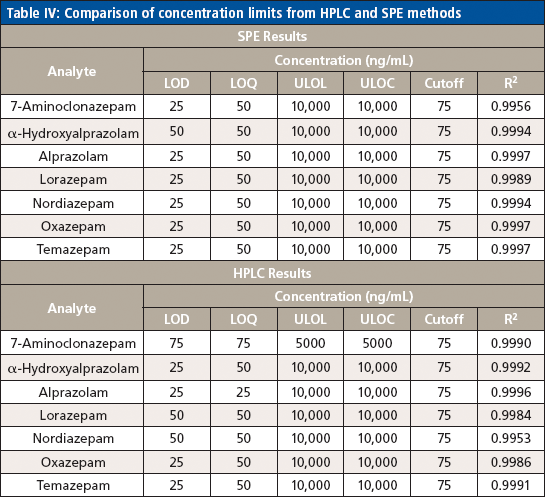
Table IV: Comparison of concentration limits from HPLC and SPE methods
The LLOQ was identified by the analysis of calibration curves in five replicates. It was defined as the lowest non-zero calibrator with acceptable ion ratio and suitable precision (<15% CV) and accuracy (±25%) included in the calibration curve that gave an acceptable coefficient of determination (R2 ≥ 0.99). The RF LLOQ was 50 ng/mL, and the LOD was 25 ng/mL. The ULOQ was set to 10,000 ng/mL based on the therapeutic range.
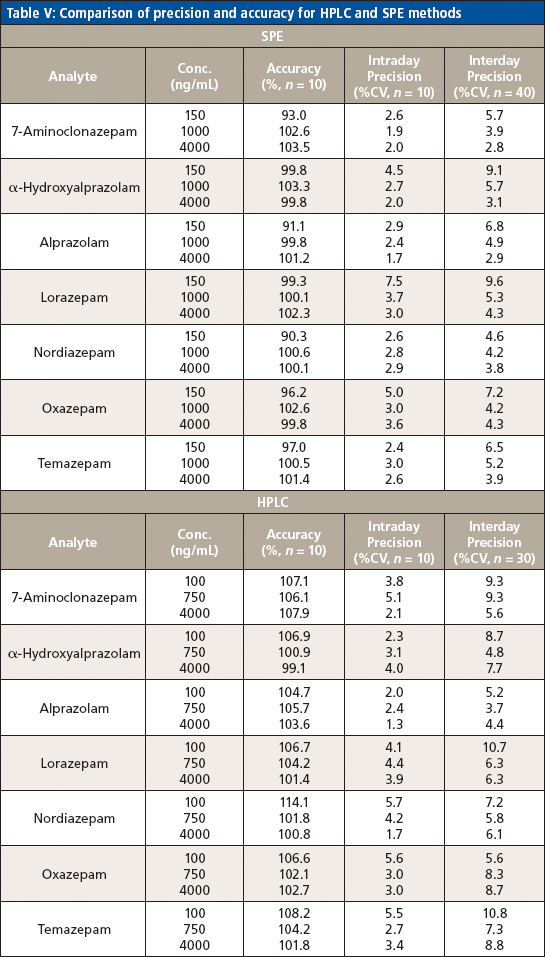
Table V: Comparison of precision and accuracy for HPLC and SPE methods
The absence of carryover was demonstrated by injecting a negative urine sample after a sample spiked with 10,000 ng/mL of each analyte. Carryover was considered insignificant if none of the analytes was detected at or above the LLOQ. This method was free from carryover at 10,000 ng/mL.
The matrix effect on each analyte was assessed by comparing the relative response ratio of each compound prepared in neat urine as well as in water at a mid-level concentration in the calibration curve. Five replicates of each analyte in each preparation solution were run. The average relative response ratio was determined for each set of samples and the matrix effect was calculated as follows: % matrix effect = ([average matrix response – average water response]/average water response) × 100. A positive result indicated ion enhancement, and a negative result showed ion suppression. Results are summarized in Table VI. Both alprazolam and temazepam demonstrated large matrix effects, which did not appear to affect the limitations of the method.
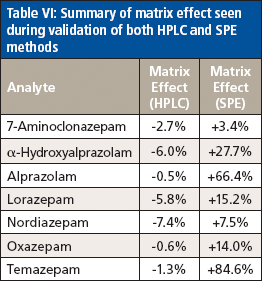
Table VI: Summary of matrix effect seen during validation of both HPLC and SPE methods
Interference from endogenous matrix components, the internal standard, and other possible drug compounds was determined through a variety of studies. To rule out interference on the internal standard, five replicates of each individual analyte were prepared at the ULOQ, substituting water for the internal standard. No interference was detected at the internal standard retention time. Matrix interference was excluded by analyzing five replicates of neat urine with no internal standard. Potential interference from other drug compounds was assessed by examining the responses of a variety of related pain medications. None of the analytes of interest were detected above the LLOQ in any of these tests. Because the automated SPE method also eliminates chromatography, an additional interference study was performed to determine if any of the possible interfering drug compounds contributed to significant ion suppression of the analytes of interest. Each benzodiazepine analyte in the new method was made up individually at a mid-level concentration (1000 ng/mL) and spiked into the various interference drug mixes containing compounds at extremely high concentrations. None of the potential interfering compounds demonstrated a suppressive effect on any of the benzodiazepines.
Precision (intra- and inter-run) and accuracy were determined by examining 10 replicates of each of the three concentrations across the calibration range over the course of three days and calculating the percent of target for accuracy and percent CV for precision. Acceptable accuracy (<14%) and intra- and inter-run precision (<11% CV) were found at each concentration for each analyte of interest.
A comparison of patient samples was performed to assess how the results correlated between the automated SPE method and the production HPLC method. First, 60 patient samples that were positive for a variety of the benzodiazepines of interest were analyzed by LC–MS-MS, then diluted 20-fold and analyzed by SPE–MS-MS. The data between the two methods correlated well (percent deviation did not exceed 30%), but peak interferences were observed for nordiazepam and temazepam in patient samples. While the nature of these interferences is under investigation, it was found that the use of an exact mass, high-resolution QTOF MS system eliminated the interferences from these samples. Figure 2 (top) shows the response for a sample with the interferent using a triple-quadrupole detector. This sample was not positive for nordiazepam by the current HPLC–MS-MS method, yet a positive peak is clearly visible using the automated SPE-MS-MS method. Figure 2 (bottom) shows the same sample with detection via the QTOF system. Note the total absence of signal between 8 s and 12 s of the elution time exactly where the "false" peak is eluted. Thus, the exact mass resolution of the QTOF system results in the absence of signal for this transition (271.1→139.9) and shows the absence of the false positive peak seen with the HPLC–MS-MS method. Migration of this method from triple-quadrupole MS to exact mass MS maintained the validation criteria observed in the validation of this method.
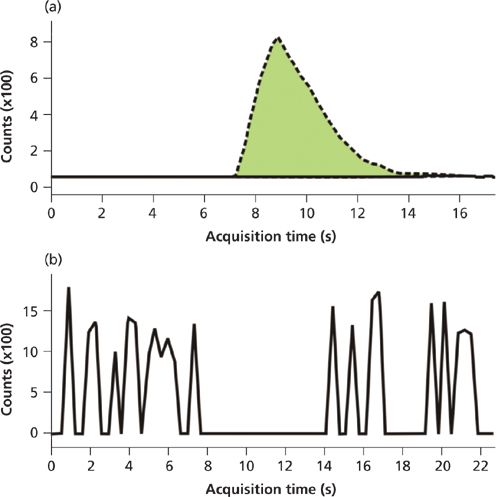
Figure 2: (a) A chromatogram depicts the nordiazepam interference (false positive) seen when using a triple-quadrupole MS system with the on-line SPE system for benzodiazepine detection in patient samples. (b) The same sample injected into a QTOF shows the lack of a peak (that is, noise) for nordiazepam, demonstrating exact mass resolution of the interferent.
Discussion
Aside from opiate testing, benzodiazepine panels account for the predominant bulk of all samples tested in the clinical pain management laboratory, especially in a pain medication monitoring facility. Because of the volume of the panel, a faster, more efficient, and ideally cheaper method was desired. The existing HPLC–MS-MS method was multiplexed to allow for a 96-s sample injection to sample injection cycle time. On-line sample cleanup makes injection of enzyme-containing samples possible without preceding enzyme removal. Although this method appears fairly rapid, consumable costs (columns, internal standards, and solvents) were mounting, and other options to increase efficiency were explored.
An SPE-based front-end system was selected because of its speed in sample introduction. With a cycle time of 13 s from sample injection to sample injection, the method developed on the SPE system allowed for a sevenfold increase in throughput while using a smaller number of MS instruments to handle the volume. The cost savings were also significant because of SPE cartridge lifetime and lower overall operating cost, leading to at least a 10-fold savings over the traditional HPLC–MS-MS analysis. The cartridge lifetime is notable in spite of simply diluting the enzyme-containing samples before injection. It could reasonably be expected that enzymes or proteins may clog or otherwise adversely impact the efficiency of the cartridge much like an analytical column. However, the design of the SPE cartridge, with its relatively large particle size, enables this type of dilute-and-shoot methodology.
Method development and validation were performed as described above using seven compounds of interest (7-aminoclonazepam, α-hydroxyalprazolam, alprazolam, lorazepam, nordiazepam, oxazepam, and temazepam) and one internal standard (nordiazepam-D5), with two transitions monitored for each compound. The use of a single internal standard resulted from limitations associated with the lack of chromatography and the number of total transitions the mass spectrometer can monitor at a single time while retaining enough points across each peak to ensure peak fidelity. For similar reasons, dwell times on the triple-quadrupole and QTOF-MS systems had to be carefully monitored. Data from the method validation demonstrated comparable responses and quantitation when compared to the HPLC–MS-MS method where an internal standard was used for each compound, leading to the conclusion that only one internal standard was sufficient for accurate results. The use of a single internal standard contributed to the decrease in cost observed in the transition to the new SPE–MS-MS method.
Interestingly, all analytes demonstrated positive matrix effects, with those for alprazolam and temazepam as the most significant. It is possible that the urine matrix assists the adsorption onto the SPE cartridge and thus results in increased responses relative to those seen in water (neat preparation) alone. Salt content and pH would be expected to have an impact on partitioning onto the SPE and inasmuch as these samples are all buffered for enzyme efficiency, it would seem to point to increased salt content as the driving force in this enhanced response.
Comparison of patient data obtained using the SPE–MS-MS method to data obtained using the conventional HPLC–MS-MS method demonstrates good correlation, despite a cycle time that is seven times shorter. However, it is worth noting that the elimination of chromatography with the SPE–MS-MS method led to the appearance of an interferent in patient samples for the nordiazepam and temazepam transitions. Patients who were observed positive for those two compounds by HPLC–MS-MS were also observed positive by SPE–MS-MS; however, patients that had not previously demonstrated a response for either compound by HPLC–MS-MS did show one when analyzed by SPE–MS-MS. Although these "false positives" were not in fact positives by quality control criteria (ion ratios), the appearance of the interferent casts doubt on the confirmation of those two compounds and implies that the SPE–MS-MS method cannot be used in a production environment with a triple-quadrupole detector.
Interestingly, use of a QTOF detector resolves these interferences and offers a way forward for this method. Even though initial parent ion selection on a QTOF system has the same mass resolution limitation as a triple-quadrupole system (approximately 1 Da), the use of an exact mass TOF analyzer on the back end allows for more definitive detection. This effectively removes the false fragment selection, thus eliminating the interference. As illustrated in Figure 2, the exact mass detector offered clean peaks without the interferent observed with the triple-quadrupole instrument. Repeat studies with calibrators demonstrated equivalent LLOQ and LOD values, as well as dynamic range and precision.
Conclusion
The newly developed SPE–MS-MS method for the analysis of select benzodiazepine compounds returns results comparable to those found by the traditional HPLC–MS-MS method, but with a much faster analysis time and at a much lower cost per sample. Despite the absence of chromatography in the SPE–MS-MS method, the limits of analysis for the benzodiazepine panel were retained or improved on when compared to the HPLC method; however, though patient samples correlated well, peak interferences were observed for two of the compounds, precluding the SPE–triple-quadrupole MS method from entering the production workflow. The use of the QTOF detector with the SPE system showed the interferences can be resolved, and this SPE–MS-MS method may prove to be a good alternative to HPLC–MS-MS for the analysis of select benzodiazepine compounds, thereby greatly increasing efficiency while reducing cost.
Funding
All funding was provided by Ameritox, Ltd.
Acknowledgments
The authors wish to thank Dr. Michelle Romm of Agilent Technologies, as well as Dr. Maxine Jonas, for their expertise and assistance with method development on the RapidFire system.
Jennifer C. Hitchcock, Jeffrey R. Enders, Ayodele A. Morris, and Gregory L. McIntire are with Ameritox, Ltd., in Greensboro, North Carolina. Direct correspondence to: greg.mcintire@ameritox.com
References
(1) M. Lader, Journal of Substance Abuse Treatment8, 53–59 (1991).
(2) S.C. Licata and J.K. Rowlett, Pharmacol. Biochem. Behav. 90(1), 74–89 (2008).
(3) R.C. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 7th ed. (Biomedical Publications, Foster City, California, 2004), pp. 45, 376, 471, 940, 1251, and 1621.
(4) S. Marriott and P. Tyrer, Drug Safety9(2), 93–103 (1993).
(5) M. Bartholow, Pharmacy Times 79(7) (2013). www.pharmacytimes.com/publications/issue/2013/July2013/Top-200-Drugs-of-2012. Accessed January 2014.
(6) L.P. Longo and B. Johnson, Am. Fam. Physician 61(7), 2121–2128 (2000).
(7) C.H. Ashton, Benzodiazepine Abuse. Drugs and Dependence, (Harwood Academic Publishers, New York, New York, 2002), pp. 197–212.

Best of the Week: Exclusive on Flow Imaging Microscopy, Interview with PNNL Chief Science Officer
April 4th 2025Top articles published this week include several interviews with key opinion leaders on various topics including advanced mass spectrometry (MS) technologies in studying diseases, microplastic detection, and interpreting Raman spectra.
New Multi-Spectroscopic System Enhances Cultural Heritage Analysis
April 2nd 2025A new study published in Talanta introduces SYSPECTRAL, a portable multi-spectroscopic system that can conduct non-invasive, in situ chemical analysis of cultural heritage materials by integrating LIBS, LIF, Raman, and reflectance spectroscopy into a single compact device.