Rapid Classification of Beef Aroma Quality Using SIFT-MS
Special Issues
Application of multivariate statistical analysis to a combined data set demonstrated that SIFT-MS discriminates premium quality beef from eight sensory defects, and, therefore, could be applied as an instrumental grading tool, obviating sensory panel grading.
Aroma is an important characteristic in the acceptance of beef by consumers, and preference is often culturally dependent. Traditional grading of aroma quality has been carried out using sensory analysis of very limited numbers of samples, due to the inherent costs and the lack of appropriate technologies to replace the human olfactory system. Since meat aroma is derived from various volatile organic compounds (VOCs) that impart favorable or unfavorable qualities, there is potential to apply selected ion flow tube mass spectrometry (SIFT–MS) to facilitate wider scale, economic grading of carcasses. SIFT–MS is a direct analysis technique that can provide both high sample throughput and selective analysis of the chemically diverse volatiles that contribute to the aroma. This paper describes an investigation of the applicability of SIFT–MS to beef grading. Classifications of beef aroma were provided by a trained sensory panel. SIFT–MS was used to analyze the same samples for aroma volatiles. Application of multivariate statistical analysis to the combined data set demonstrated that SIFT–MS discriminates premium quality beef from eight sensory defects, and, therefore, could be applied as an instrumental grading tool obviating sensory panel grading.
There are several factors that contribute to the acceptability of a beef cut to the consumer, but most important is its flavor. Certain volatile organic compounds (VOCs) impart favorable or unfavorable characteristics to the flavor, and these can be detected using a variety of analytical techniques. Beef flavor is made up from a significant number of volatile compounds, largely arising from the cooking process. These flavor compounds are generated in the Maillard reaction, lipid oxidation, and interactions between them during the cooking process (1–7), and from vitamin degradation (1,8). A wide range of volatile flavor compounds are produced during the cooking of beef, and, if an understanding of the flavor compounds is to be found, many different volatile compounds should be monitored. Further, the aroma of cooked beef can be complex, and many hundreds of volatile flavor compounds have been identified (2,3,6,9-13). For example, a list of 90 VOCs have been monitored as flavor compounds in cooked beef, using the technique of gas chromatography–olfactometry (GC/O) (2,14). These compounds include aldehydes, ketones, alcohols, hydrocarbons, and pyrazines. Many of the traditional techniques for monitoring beef flavors are based on gas chromatography-mass spectrometry (GC–MS), often in conjunction with olfactometry. However, analysis using gas chromatographic techniques are slow and require expert operation, so they are impractical for process applications.

Recently, direct mass spectrometric methods have been developed that eliminate time-consuming chromatographic analysis. In this paper, we apply one of these methods: Selected Ion Flow Tube Mass Spectrometry (SIFT–MS), a rapid, highly sensitive analyzer of whole air, to the detection of VOCs from various New Zealand beef samples. The rapid analysis provided by SIFT-MS has the potential to quickly identify premium quality carcasses early in the production process.
Methods
SIFT–MS
SIFT–MS (15,16) is a real-time analytical technique for direct, comprehensive gas analysis to ultra-trace levels (17). Data obtained by SIFT–MS instruments compare well with the leading chromatographic method for volatile organic compound (VOC) analysis, GC–MS (18).
SIFT–MS uses soft, precisely controlled chemical ionization, coupled with mass spectrometric detection (Figure 1), to rapidly quantify VOCs to low part-per-trillion concentrations by volume (pptv). Eight chemical ionization agents (reagent ions) are now available in commercial SIFT–MS instruments: H3O+, NO+, O2+, O-, O2-, OH-, NO2-, and NO3- (19). These reagent ions react with VOCs and inorganic gases in well controlled ion-molecule reactions, but they do not react with the major components of air (N2, O2, and Ar). This enables SIFT–MS to analyze air at trace and ultra-trace levels without pre-concentration.
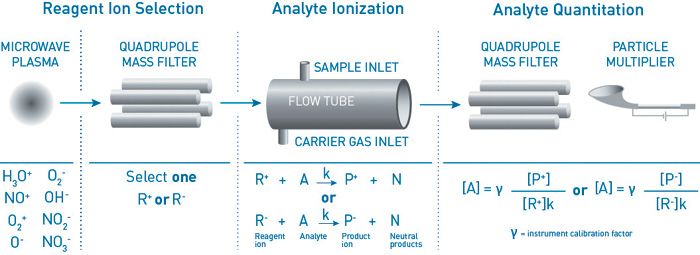
Figure 1: Schematic diagram of SIFT-MS – a direct chemical-ionization analytical technique.
Rapid switching between the eight reagent ions provides very high selectivity. The key benefit of the additional ions is not primarily in the number of reagents ions, but in the multiple reaction mechanisms that provide additional independent measurements of each compound, delivering unparalleled selectivity and detection of an extremely broad range of compounds in real time.
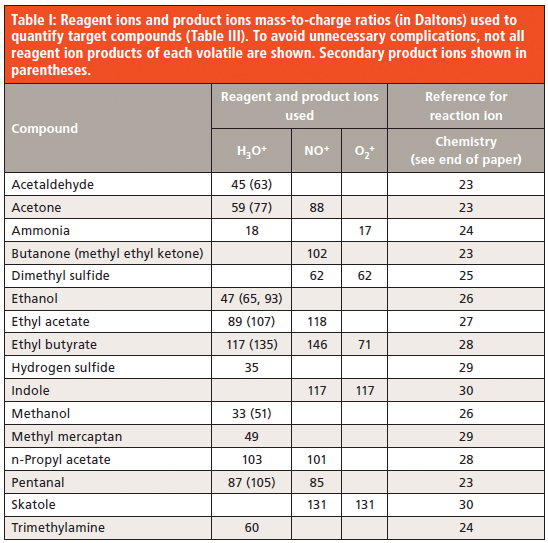
In this paper, a Voice200 SIFT–MS instrument (Syft Technologies, Christchurch, New Zealand; www.syft.com) was utilized in selected ion mode (SIM). The compounds targeted together with the masses used for quantitation are summarized in Table 1. The three standard positively charged reagent ions were utilized to achieve selective analysis.
Samples
Sensory profiling
To create a range of flavor profiles, samples were sourced either from different types of cattle, or by modifying processing and storage conditions for vacuum packet primal cuts:
- normal production of prime grass-fed cattle
- high pH beef from both prime and bull carcasses
- manipulation of product by additional aging/chilling at higher temperatures to develop undesirable storage-related flavors
- sourcing product with abnormal flavors from both bulls and cows.
Previous studies have shown that beef from older bull and cow animals can be associated with "barnyard" and "milk" notes, respectively. Beef with an elevated final pH (high pH) relates to carcasses that have insufficient glycogen at the point of slaughter to enable the normal post mortem glycolytic cycle to progress. This condition has been associated with "sour" or metallic-like notes. Therefore, samples with these attributes were included in the sample selection provided by Carne Technologies (Table 2).
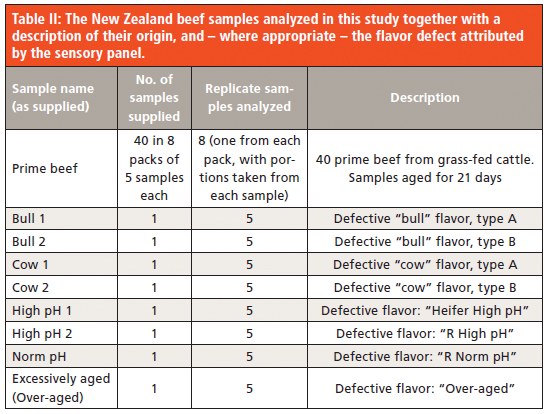
Samples were collected and stored frozen prior to sensory analysis, then thawed and minced prior to cooking and serving. Sensory panelists trained by Carne Technologies to recognize and assess aromas and flavors were used to evaluate each sample. The attributes used by the sensory panelists consisted of meaty, beefy, barnyard, sour/acidic, milky, grassy/pasture, sweetness, offal, spoilage, rancid, storage, other/foreign (such as garlic or pepper), and aftertaste. Each attribute was scored on a 9-point scale where 0 is absent and 9 is intense/extreme. All samples, except "prime beef," exhibited different sensory defects.
Preparation for instrumental analysis
For SIFT–MS analysis, frozen samples were thawed at room temperature and finely diced. Samples of uncooked beef (20 grams) were placed in one-liter Schott bottles and capped with pierceable septa. Samples were incubated at 60 °C for one hour prior to analysis, using a SIFT-MS instrument equipped with a high-performance inlet (HPI) that is both passivated and heated (120 °C). The HPI provides a direct path from the sample headspace to the flow tube, minimizing loss of flavor volatiles.
Multivariate Statistical Analysis
The Selected Ion Mode (SIM) data from SIFT–MS were treated using multivariate statistical analysis to determine the ability of SIFT–MS to discriminate between the premium samples labelled "prime beef" and samples deemed defective by the sensory panel.
The multivariate statistical methodology applied in this work was Soft Independent Modeling by Class Analogy (SIMCA), which was developed by Wold in the 1970s (20). SIMCA applies principal component analysis (PCA) to the whole dataset and to each of the classes with the goal of creating a model that discriminates each class from the others. The Infometrix Inc. (Bothell, WA) implementation of the SIMCA algorithm in the Pirouette software package was employed here.
Three types of output from the SIMCA analysis are presented in this report:
- Class projections: These three-dimensional plots show how each sample falls with respect to the three most important principal components derived from PCA on the entire data set. Each user-defined class shows the sample with the same color and a "cloud" representing the calculated space in which all samples of the class are expected to lie. Better class separations lead to more confident assignment of unknown samples to a predefined class, if a suitable one exists.
- Interclass distances: These are a measure of the separation between classes, a value of three usually being considered acceptable for class separation (21). Sometimes, the class separability indicated by these distances is not apparent in the three-dimensional class projection plot.
- Discriminating power: This parameter helps identify variables that provide the most discrimination between the classes. A variable with larger discriminating power has greater influence on separating the classes than one with a small discriminating power. There does not appear to be a set threshold value above which this variable is considered good, because these values vary strongly with interclass distance.
Results and Discussion
The data obtained from SIFT–MS analysis of the headspace of New Zealand beef samples are summarized in Table 3. For each class of sample, the replicate measurements have been averaged and the extent of variation indicated using two standard deviations of the mean. For most compounds, measurements show satisfactory repeatability given that analysis was carried out manually (since undertaking this study, application of automation to SIFT–MS has demonstrated significant repeatability improvements compared to manual analysis [22]). Limits of quantitation are typically below one part-per-billion by volume (ppbv) for headspace analysis using SIFT–MS. Direct analysis using soft chemical ionization means that all compounds were analyzed in a single, two-minute run for each sample. SIFT–MS requires no preconcentration or sample dilution, due to its wide dynamic range and its high robustness to moisture.
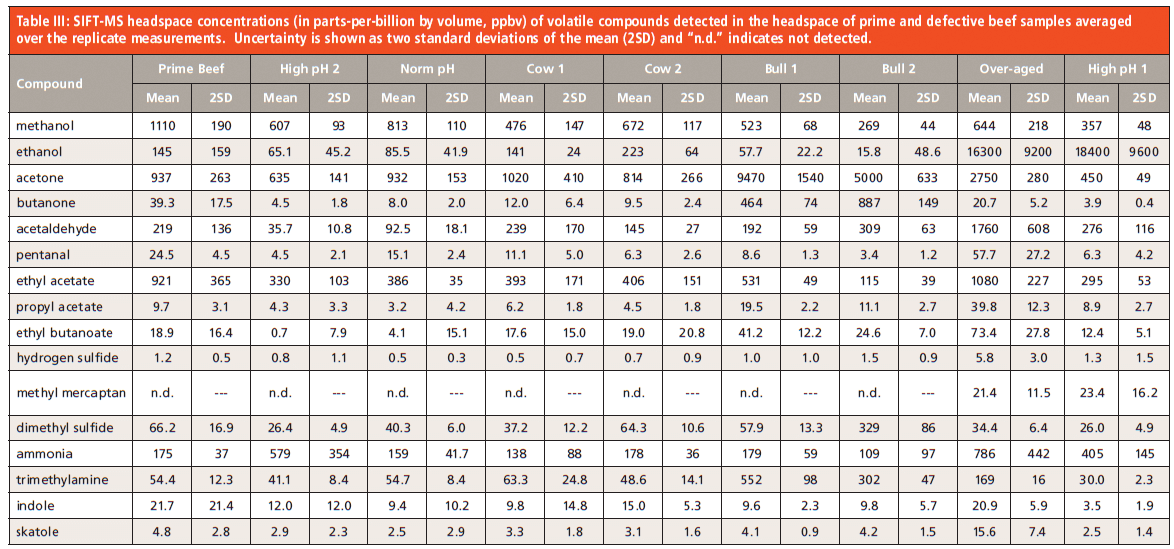
The top-rated prime beef samples generally lie in the mid-range of concentration values for the compounds targeted here, except for ethyl acetate, indole, and methanol, which are at the upper end. The repeatability observed for these premium steaks is particularly pleasing, because it illustrates that there is great consistency across the 40 animals that they were obtained from.
Defective beef samples tend to deviate significantly for several target compounds compared to the premium cuts. For example, the samples labeled "over-aged" and two of the "high pH" samples have elevated ammonia concentrations. The over-aged sample has elevated hydrogen sulfide and methyl mercaptan, both of which are extremely pungent; the latter is also detected in the "High pH 1" sample. This means that there is some potential for detecting particular defects by applying thresholds for a handful of markers.
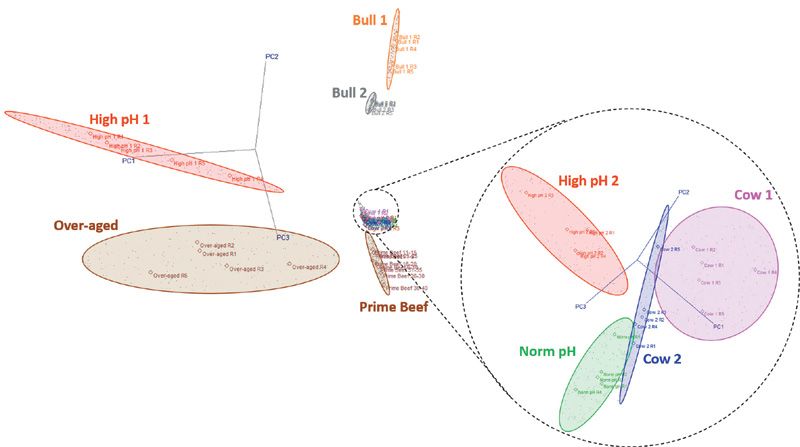
Figure 2: Class projections obtained from SIMCA multivariate analysis of the headspace data that are presented in averaged form in Table III. Each colored point in the class projections graph represents a replicate measurement. For clarity, the more congested region is expanded and rotated at right.
However, certain defects (such as those found in the "Cow 1" and "Cow 2" samples) are not so readily distinguished from premium steaks based on thresholds of several marker compounds. Application of multivariate statistical analysis to the concentration data can be applied to achieve this. Figure 2 shows the class projections obtained when the full data set is processed using the SIMCA algorithm. Each colored point in this plot represents a replicate measurement. For clarity, the more congested region is expanded on the right-hand side of Figure 2. Table 4 summarizes the interclass distances obtained from the SIMCA analysis: all beef classes in this study are separable since the distances are all greater than three (21), including those that look marginal in the class projections plot ("Norm pH", "Cow 1", "Cow 2").
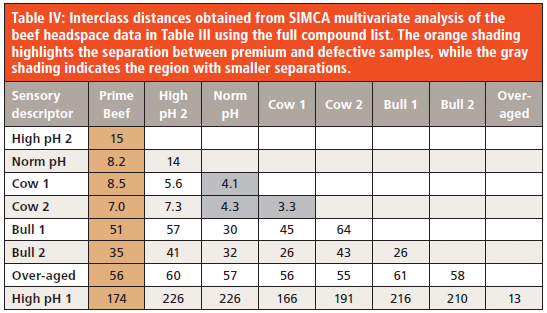
Table 5 summarizes the relative significance of various target compounds in discrimination of the beef samples: the larger the number, the greater the contribution. Ethanol dominates discrimination. Given that the flavor impact of ethanol is lower than the other target compounds, and that ethanol was the key discriminating volatile between grass versus pellet fed cattle (from a parallel, unpublished study), the data was reprocessed using the SIMCA algorithm with both ethanol and methanol removed (methanol was the second most significant discriminator in the feed study). The right-hand column of Table 5 lists the discriminating powers with this revised compound list, while Table 6 gives the interclass distances. The prime beef samples remain well separated from the defective samples, as do most of the defects from each other. However, the two "cow" samples ("Cow 1" and "Cow 2") are no longer completely separable with an interclass distance of 2.3, and "Cow 1" is not entirely distinguishable from the sample labelled "Norm pH".
Conclusion
The results presented here demonstrate that the SIFT-MS technique can effectively discriminate between prime and defective beef flavors when coupled with multivariate statistical analysis. The laboratory-based approach used here enables throughputs of about 30 samples/hour to be achieved. Even in its present form, SIFT–MS shows promise for wider scale flavor quality testing off-line compared to the traditional sensory approach.
The goal, however, is to take this demonstrated flavor-screening potential to an in-line or near-line application. To achieve this, sample throughput needs to be enhanced twentyfold, to meet the needs of modern beef processing lines. This requires both optimization of the SIFT-MS analytical method and rapid heating of the beef sample to facilitate faster release of flavor compounds. A possible rapid sampling approach could utilize the i-knife, which was invented for surgical applications, but has recently been evaluated for meat (31).
Author Contributions
Nicola Simmons, Clyde Daly, and Vaughan Langford designed the experiments. The late Tracey Cummings coordinated sensory analysis and selected samples for instrumental analysis. Langford conducted the instrumental analysis and processed the data. Langford and Murray McEwan wrote this article.
REFERENCES
(1) J.S. Elmore, D.S. Mottram, in Improving the Sensory and Nutritional Quality of Fresh Meat, J.P. Kerry, D. Ledwood, Eds. (Woodhead Publishing, Cambridge, UK, 2009), pp. 111–146.
(2) H. Van Ba, I. Hwang, D. Jeong, and A. Touseef, Latest Research into Quality Control, (InTechOpen, 2012), 145–176.
(3) K. Specht and W. Baltes, J. Agric. Food Chem. 42, 2246–2253 (1994).
(4) J. Kerler and W. Grosch, J. Agric. Food Chem. 53, 9578–9585 (2005).
(5) R. Tansawat, C.A.J. Maughan, R.E. Ward, S. Martini, and D.P. Cornforth, Int. J. Food Sci. Technol. 48, 484–495. (2013).
(6) J.S. Elmore, D.S. Mottram, M. Enser, and J.D.J. Wood, J. Agric. Food Chem. 47, 1619–1625 (1999).
(7) D.S. Mottram, Food Chem.62, 415–424 (1998).
(8) G. Reineccius, Flavor Chemistry and Technology (Taylor & Francis, Abingdon, UK, 2nd Ed., 2006).
(9) U. Gasser and W. Grosch, Z. Lebensm. Unters. Forsh. 186, 489–494 (1988).
(10) S. Rochat and A. Chaintreau, J. Agric. Food Chem. 53, 9578-9585 (2005).
(11) D. Machiels, L. Istasse, and S.M. van Ruth, Food Chem. 86, 377-383 (2004).
(12) J. Kerler and W. Grosch, Journal Food Sci.61, 1271- 1274 (1996).
(13) H. Van Ba, M. Oliveros, K.S. Ryu, and L. Hwang, J. Food Science of Animal Resource 30, 73-86 (2010).
(14) Y. Takakura, T. Sakamoto, S. Hirai, T. Masuzawa, H. Wakabayashi, and T. Nishimura, Meat Science, 97, 27–31 (2014).
(15) P. Spanel and D. Smith, Med. & Biol. Eng. & Comput. 24, 409–419 (1996).
(16) D. Smith and P. Spanel, Mass Spec. Rev. 24, 661–700 (2005).
(17) B.J. Prince, D.B. Milligan, and M.J. McEwan, Rapid Commun. Mass Spectrom. 24, 1763–1769 (2010).
(18) V.S. Langford, I. Graves, and M.J. McEwan, Rapid Commun. Mass Spectrom. 28, 10–18 (2014).
(19) D. Hera, V.S. Langford, M.J. McEwan, T.I. McKellar, and D.B. Milligan, Environments, 4, 16 (2017).
(20) S. Wold, Pattern Recognition, 8, 127–139 (1976).
(21) O.M. Kvalheim and T.V. Karstang, in Multivariate Pattern Recognition in Chemometrics, Illustrated by Case Studies, R.G. Brereton, Ed. (Elsevier: Amsterdam, 1992), pp. 209–238.
(22) M.J. Perkins, V.S. Langford, and M.J. McEwan, Current Trends in Mass Spectrom. 16, 24–29, 2018.
(23) P. Spanel, Y. Ji, and D. Smith, Int. J. Mass Spectrom. Ion Proc. 165/166, 25–37 (1997).
(24) P. Spanel and D. Smith, Int. J. Mass Spectrom. 176, 203–211 (1998).
(25) P. Spanel and D. Smith, Int. J. Mass Spectrom. 176, 167–176 (1998).
(26) P. Spanel and D. Smith, Int. J. Mass Spectrom. Ion Proc. 167/168, 375–388 (1997).
(27) P. Spanel and D. Smith, Int. J. Mass Spectrom. 172, 137–147 (1998).
(28) G.J. Francis, D.B. Milligan, and M.J. McEwan, J. Phys. Chem. A 111, 9670–9679 (2007).
(29) T.L. Williams, N.G. Adams, and L.M. Babcock, Int. J. Mass Spectrom. 172, 149–159 (1998).
(30) T. Wang, P. Spanel, and D. Smith, Int. J. Mass Spectrom. 237, 167–174 (1998).
(31) J. Balog, D. Perenyi, C. Guallar-Hoyas, A. Egri, S.D. Pringle, S.Stead, O.P. Chevallier, C.T. Elliot, and Z. Takats, J. Agric. Food Chem. 64, 4793–4800 (2016).
Vaughan S. Langford is with Syft Technologies, Ltd. in Christchurch, New Zealand. Murray J. McEwan is with Syft Technologies, Ltd. in Christchurch, New Zealand and the Department of Chemistry at the University of Canterbury in Christchurch, New Zealand. Tracey Cummings, Nicola Simmons, and Clyde Daly are with Carne Technologies Ltd. in Cambridge, New Zealand. Direct correspondence to Vaughan.Langford@syft.com

Best of the Week: Flow Imaging Microscopy, Electric Vehicles, Air Pollution in India
April 11th 2025Top articles published this week include an interview about flow imaging microscopy, a news story on fiber optics and electric vehicles, and a recap of a study that explored the use of micro-particle-induced X-ray emission (micro-PIXE) spectroscopy to trace urban and indoor air pollution sources in India.