Recent Developments in Small-Angle X-Ray Scattering
Special Issues
There has been a resurgence in the application of small-angle X-ray scattering for a large range of problems in materials science. This article highlights experimental requirements and applications, with examples drawn from protein solutions, porous structures, and polymers.
With the availability of high intensity synchrotron sources, fast electronic detectors, and computational resources, there has been a resurgence in the application of small-angle X-ray scattering for a large range of problems in materials science. Some of these developments are discussed using examples drawn from porous structures, protein solution, and polymers and fibers. New analytical techniques such as Monte Carlo simulations of solution scattering data and full pattern analysis of the diffraction data from lamellar structures are also described. Finally, some of the online resources available for such analysis are provided.
With the availability of synchrotron X-ray sources, fast two-dimensional (2D) electronic detectors, and enhanced computational capabilities, there has been a resurgence in the use of small-angle X-ray scattering (SAXS) in biology and materials science. The technique is now more accessible and has become an indispensable analytical tool for studying statistically representative microstructures of heterogeneous materials at 10–1000 nm length scales (1).
The ability of X-rays to be absorbed, fluoresce, and diffract is used to examine materials by imaging, spectroscopy, and crystallography, respectively. SAXS, on the other hand, measures the coherent scattering of X-rays at small angles to the primary beam (<2° with copper Kα radiation; 1.54 Å wavelength) to probe the structure at the meso-length scales that commonly occur in biology and materials science. This capability includes measuring the shapes and sizes of nanoparticles and large molecules, domains and voids, organized structures at large length scales, and, in general, electron density fluctuations and inhomogeneities with characteristic dimensions between 10 and a few hundred nanometers (2). SAXS complements other techniques such as microtomography, electron microscopy, and atomic force microscopy (AFM). When neutrons are substituted for X-rays, the resulting small-angle neutron scattering (SANS) data can extend the utility of small-angle scattering (SAS) by being able to adjust the contrast based on the nuclear structure rather than the electron density differences as in X-rays (3).
The areas in biology and materials science that can benefit by using SAXS fall into two broad categories: particulate systems (dilute and crowded particles) and nonparticulate systems (random two-phase and ordered structures). Systems with dilute particles include proteins and polymers in solution, colloids, defects in crystals, and inclusions in solids and voids (porous materials). Examples of dense particulate systems with long-range organization are colloidal aggregates, clusters of nanoparticles, microemulsions, and fractal objects. Solid two-phase systems include polymers, catalysts, nanocomposites, porous media, metallic alloys, and other microphase separated systems. Finally, examples of ordered structures (self-assembled systems) can be found in biological structures, semicrystalline polymers, block copolymers, fibers, liquid crystalline materials, and nanostructures. This article highlights experimental requirements and applications, with examples drawn from protein solutions, porous structures, and polymers.
The SAXS Method
The SAXS experiment is conceptually simple. The sample is illuminated by X-rays, and the scattered radiation is registered by a detector. Because the SAXS measurements are done very close to the primary beam, at small angles, collimation of the beam is stringent and the instrument requires long flight paths (on the order of meters). Consequently, essential elements of the instrument are proper alignment of the numerous slits to define the beam, containment of the sample in a cell with minimum scatter, the smallest possible beam stop that does not contribute to stray scattering, and an evaluated beam path.
Until the 1980s, SAXS experiments were carried out using instruments equipped with laboratory X-ray tubes. But higher intensity sources are required to compensate for the loss in intensity resulting from fine collimation and the long flight path. Synchrotron sources reduce the time of SAXS data collection from as long as 24 h to seconds, or even shorter. Additionally, these sources make it possible to develop hyphenated techniques by combining X-ray scattering with differential scanning calorimetry (DSC) (4), infrared (IR) or Raman spectroscopy, electrochemistry, fluorescence, X-ray spectroscopy (5), dynamic mechanical analysis, and, more recently, computed tomography (6). It is now possible to routinely carry out time-resolved experiments to study the influence of temperature and deformation to understand the physical mechanisms behind macroscopic phenomena such as thermal expansion and physical deformation by monitoring the motion of the molecular chains. Microdiffraction with beams of ~0.5 µm (7,8) can be used to characterize spatial distribution of defects at polymer interfaces, nanostructures in fiber-reinforced composites and high-performance fibers, interfaces, and skin-core morphologies.
Applications of SAXS
Scattering from unoriented structures are analyzed by reducing the observed data into one-dimensional (1D) scans such as the one shown in Figure 1. The various regions in the patterns are indicated in the figure. Such 1D data are typically analyzed by using approximations (such as Guinier and Porod) that are valid over a limited angular range, correlation, or pair-wise distribution functions, and by modeling (2). Scattering from oriented structures needs to be analyzed using 2D data of the type shown in Figure 2. Such patterns can be used to gain valuable insight into the relation between structure and deformation. These 2D patterns can be analyzed by full pattern fitting. In many instances, such 2D patterns fall on an elliptical grid, and therefore are best analyzed in elliptical coordinates (9,10).
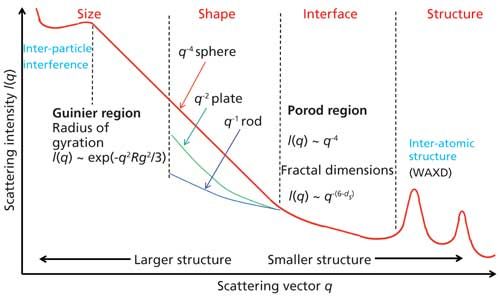
Figure 1: Schematic of 1D SAXS scan from a particulate system. The intensity is plotted as a function of scattering vector q, which is related to the scattering angle 2Ï´ by the relation q = (4π sinÏ´/λ), where λ is the wavelength of the X-rays. The three commonly recognized regions of the SAXS regions are indicated in the figure.
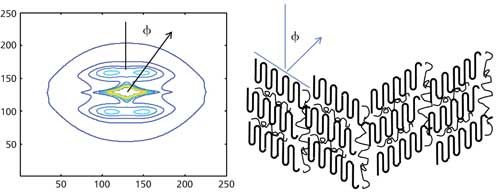
Figure 2: SAXS data in the form of contour plots from an ordered condensed phase. The data were fitted in elliptical coordinates to two functions corresponding to the lamellar peak and the equatorial streak (11). A schematic of the tilted lamellae that could give rise to such a pattern is shown on the right.
The central diffuse scattering that arises from dilute systems (Figure 1) is analyzed to obtain particle parameters such as the radius of gyration (Rg) from Guinier plots, molecular weight from I(0), compactness of the molecule from Kratky plots, surface area, volume, and surface-to-volume ratio and fractal dimensions (ds) from Porod plots (2,12). Immense activity to probe the behavior of proteins in solution has led to the advent of robot-controlled, stream-lined data collection at the synchrotron sources and automated high-throughput analysis (13,14). More recently, biomolecule structures are being modeled with one-bead-per-residue representation (course-grain model), as a set of spherical structures, or composed of several candidate structures that are aligned and subsequently clustered to reproduce the observed data. In these modeling efforts, SAXS data is used to choose a subset of conformations from a large pool of candidate structures generated from prior knowledge of the interactions between the various domains or residues within the molecule. Alternatively, the scattering body is expressed as a series of spherical harmonics, and the scattered intensity is calculated as the sum of the contributions from these substructures (15). Note that fitting high-resolution structural models to low-resolution data is susceptible to overfitting.
Central diffuse scattering is also present in catalysts and ceramics, where it is used to obtain void volume fraction, pore size distributions, internal surface areas, and pore morphologies (16). Examples include zeolites, solution-mediated colloids, gels and suspensions, and for nanoparticle assemblies of soot or silica particles in flames (17). Ultra-SAXS (USAXS) is used to examine sintering, microcracking, cavitation, or creep damage on the micrometer scale. At synchrotron sources, it is possible to carry out anomalous SAXS studies by varying the energy close to an X-ray absorption edge of a specific element and thus vary the scattering contrast of one microstructure component against another.
Mesoscale ordered structures give rise to discrete reflections rather than diffuse scattering. These Bragg-like features form a 2D scattering pattern such as the one shown in Figure 2. Many biological structures such as membranes and collagen have been extensively investigated using SAXS, and continue to provide new insights into these structures (18). Ordered structures in synthetic polymers include lamellae and micelles from many polymers that crystallize, and a large number of very interesting ordered nanostructures, such as spherical micelles and cylindrical (worm-like) micelles, bilayers and vesicles, and bicontinuous structures in block copolymers. The 2D patterns of the type shown Figure 2 that arise from lamellar structures are analyzed to obtain characteristics of the lamellae such as height, width, spacing, and the orientation, which are used to study the crystallization and deformation (11,19). The results obtained using a nano-calorimeter have been especially revealing about the nature of the thermal transitions and polymer. Similarly, nanobeam diffraction has been used to study interfaces and spherulitic morphology. In addition to the lamellar reflections, there is a streak along the equator and the central diffuse scattering that contains information about the larger length scale structures such as fibrils and lamellar stacks. Both the lamellar structure and the equatorial streak are best analyzed by full-pattern fitting of the image in elliptical coordinates (9,10,20).
Software Resources
Many software packages are available online for processing, analyzing, and modeling SAXS data. Some commonly used software includes ATSAS from the European Molecular Biology Laboratory (EMBL), FIT2D and BIOSYS from the European Synchrotron Radiation Facility (ESRF), and IRENA from the Advanced Photon Source (APS). Additional information about the software and other small-angle scattering resources is available at www.smallangle.org.
Conclusion
SAXS, in contrast to the more common imaging techniques such as tomography and microscopy, can provide a view of the structure that is statistically averaged over a large sampling area. However, SAXS data, unlike the imaging techniques, appears in reciprocal-space and thus cannot provide a direct view of the structure in real-space. To address this problem, computational tools that model the structure are being developed to make the technique accessible to nonexperts. Because of these advances, coupled with remarkable developments in instrumentation, SAXS has a great potential to be widely used for investigating mesoscopic structures in diverse fields including biology, soft materials, colloids, catalysts, glasses, ceramics, and metallurgy.
Note
This article was adapted from the material that was presented at the Polymer Diffraction workshop held as a part of the 2017 Denver X-ray conference (July 31–August 4, 2017).
References
- A. Guinier and G. Fournet, Small-angle Scattering of X-Rays (Wiley, New York, New York, 1955).
- O. Glatter and O. Kratky, Small angle X-Ray Scattering (Academic Press, London, UK, 1982).
- J.E. Mark, Physical Properties of Polymers Handbook, Vol. 1076 (Springer-Verlag New York, New York, 2007).
- F. Bedoui, N. Murthy, and J. Kohn, Macromolecules 50, 2257–2266 (2017).
- W. Bras, S. Nikitenko, G. Portale, A. Beale, A. van der Eerden, and D. Detollenaere, J. Phys.: Conf. Ser.247, 1742–6596 (2010). doi:10.1088/1742-6596/247/1/012047.
- F. Schaff, M. Bech, P. Zaslansky, C. Jud, M. Liebi, M. Guizar-Sicairos, and F. Pfeiffer, Nature527, 353–357 (2015).
- C. Riekel, M. Burghammer, and M. Muller, J. Appl. Crystallogr. 33, 421–423 (2000).
- D.T. Grubb and N.S. Murthy, Macromolecules43, 1016–1027 (2009).
- N.S. Murthy, K. Zero, and D.T. Grubb, Polymer 38, 1021–1028 (1997).
- N.S. Murthy, D.T. Grubb, and K. Zero, Macromolecules 33, 1012–1021 (2000).
- N.S. Murthy and D.T. Grubb, J. Polym. Sci. Polym. Phys. 40, 691–705 (2002).
- J.E. Martin and A. Hurd, J. Appl. Crystallogr. 20, 61–78 (1987).
- M.V. Petoukhov and D.I. Svergun, Biophys. J.89, 1237–1250 (2005).
- H. Liu, A. Hexemer, and P.H. Zwart, J. Appl. Crystallogr. 45, 587–593 (2012).
- M.H. Koch, P. Vachette, and D.I. Svergun, Q. Rev. Biophys. 36, 147–227 (2003).
- H. Peterlik and P. Fratzl, Monatshefte für Chemie/Chemical Monthly137, 529–543 (2006).
- J. Hyeon-Lee, G. Beaucage, S.E. Pratsinis, and S. Vemury, Langmuir14, 5751–5756 (1998).
- A. Angelova, B. Angelov, V.M. Garamus, P. Couvreur, and S. Lesieur, J. Phys. Chem. Lett.3, 445–457 (2012).
- N.S. Murthy and D.T. Grubb, J. Polym. Sci. Polym. Phys. 41, 1538–1553 (2003).
- W. Wang, N.S. Murthy, and D.T. Grubb, J. Polym. Sci., Part B: Polym. Phys. 50, 797–804 (2012).
N. Sanjeeva Murthy is an Associate Research Professor with the Center for Biomaterials at Rutgers, the State University of New Jersey, in New Brunswick, New Jersey. Direct correspondence to: murthy@dls.rutgers.edu

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.