Spectroscopy and the Search for Ancient Life on Mars, Continued
A report published earlier this year (1) discussed a UV–vis-NIR instrument designed for use on NASA Mars rover missions. This article follows up with coverage of the Planetary Fourier Spectrometer on the European Space Agency's Mars Express orbiter mission.
Does the presence of water vapor, methane, and formaldehyde over certain regions of the Martian landscape mean that large colonies of methanogenic bacteria still are alive today? Vittorio Formisano, Istituto Fisica Spazio Interplanetario (Rome, Italy) is principal investigator for the Planetary Fourier Spectrometer (PFS) on the European Space Agency's Mars Express orbiter mission. He and his research team presented data in February 2005 that is consistent with the existence of present-day biology on Mars. Speaking to a large audience at the first Mars Express Science Conference that was held at the European Space Research and Technology Center in Norrdjiwk, The Netherlands, Dr. Formisano announced, "We have found methane and formaldehyde in the atmosphere of Mars that match up closely with a water vapor map." To put the discovery in perspective, the lifetime of methane gas in the Martian atmosphere is only 300–600 years and unless there was a source constantly replenishing the gas, it should have dissipated millions of years ago.
The actual discovery of trace amounts of methane gas (at the 11 ppb level) in the Martian atmosphere was made in 1999 by Vladimir Krasnopolsky, Catholic University of America, Washington, D.C. At the time, Krasnopolsky and his team made the observations using an Earth-based telescope located on Mauna Kea, HI, coupled to a Fourier-transform spectrometer, which placed the upper limit of methane at about 10 ppb. Krasnopolsky then said he thought the probability of the methane having a biological origin was about 70%. However, what makes the Formisano Planetary Fourier Spectrometer team discovery so important is that they have linked methane outgassing from the subsurface of Mars to its association with formaldehyde, which, according to Formisano, is an oxidation by-product of methane. The formaldehyde was found in much greater abundance than the methane, at 130 ppb. Consider, too, that formaldehyde only has a lifetime of 7.5 h in the Martian atmosphere due to untenanted UV light breaking it down and it becomes apparent that something is replenishing it at a very fast rate. The history of formaldehyde (H2CO) on Mars started with a tentative detection using the infrared spectrometer from the Russian Phobos 2 orbiter that operated for only two or three months in 1989.

Figure 1. This map shows the concentration of water vapor close to the soil around the equatorial region of Mars. The areas of least concentration are in purple, the highest in green.
Krasnolpolsky was the principal investigator of the infrared spectrometer on Phobos 2 and he some observed spectral features that indicated formaldehyde with a mixing ratio in the Martian atmosphere of 100–300 ppb. Although he was a coauthor on a scientific paper about this subject (2), Krasnopolsky was skeptical about finding much formaldehyde because it is broken down and removed by the solar UV in only 10 h on Mars. Later, he studied a high-resolution spectrum of Mars taken at the Kitt Peak National Observatory (Kitt Peak, AZ) and established an upper limit to formaldehyde on Mars at about 3 ppb. "I had heard about the detection of formaldehyde by Formisano and his team a few months ago but have not seen their spectra yet. The photochemistry of the Martian atmosphere predicts that formaldehyde is less abundant than methane by a factor of 1,000,000. Their detection means that there is a process that is more effective in the production of formaldehyde than that of methane by a factor of a few million! I don't know any natural process of this kind. I think that methanogenic bacteria are a source of methane on Mars."
Investigating the Origin of Methane and Formaldehyde
Working out the math to figure out how much methane and formaldehyde is produced, Formisano concluded that 150 tons of methane is being produced each year on Mars, while 2,500,000 tons of formaldehyde are being produced. On Earth, nearly all the methane found in the atmosphere is generated through the actions of microorganisms, which adds up to 500 million tons per year.

Figure 2. Arabia Terra is one of the three equatorial regions where the PFS instrument detected both water vapor and methane concentration. The other two areas are Elysium Planum and Arcadia Memnonia.
To make the matter even more interesting for Mars, Formisano studied water vapor maps produced by NASA's Mars Odyssey spacecraft and found that the vast reservoirs of underground water ice had a direct correlation to the location of the methane and formaldehyde. Because the methane was not distributed uniformly in the Martian atmosphere but was found only in areas where water vapor had been detected, was it possible that vast colonies of living microorganisms could be putting it there today? Formisano told New Scientist magazine in January that he felt the data pointed to life in the soil of Mars. If true, then perhaps the intriguing but ambiguous results from the 1976 Viking Labeled Release biology experiment found life after all. This will be a matter for the scientific community to judge in the near future as new discoveries are made; however, Formisano has weighed three possible explanations for the discovery of so much methane and formaldehyde on Mars.
Hot chemistry. In this scenario, active hydrothermal subsurface regions can have temperatures in excess of 400 °C, where methane then is formed from a reaction with CO2 and water. The methane eventually would outgas through the soil and into the atmosphere. Iron oxides in the soil as the methane rises then could be changed, theoretically, into formaldehyde. However, to date, there is no solid evidence for any active volcanism on Mars.
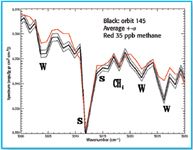
Figure 3. This "spectrum" was recorded in the areas where the methane concentration is higher. The black line shows the measured spectrum, compared to the computed methane spectrum (red line) and indicating 35 ppb of methane in the atmosphere. In other areas, the measured average of methane at Mars is only 10 ppb.
Cold chemistry. It is well known that many comets contain abundant methane and organic molecules that are thought to be produced by a reaction of solar UV and cosmic rays bombarding the water ice they contain. Perhaps Mars, like many comets, might have a similar occurrence underway.
Methanogenic bacteria. Mars has abundant microbial life 200–300 m below the soil in large aquifers.
The Planetary Fourier Spectrometer
The Mars Express Planetary Fournier Spectrometer is a highly sensitive double-pendulum infrared spectrometer specifically designed for atmospheric studies, although it can distinguish mineral compositions on the surface of Mars as well. The spectrometer is divided into two channels — a short wavelength channel and a long wavelength channel, with a resolution of 2 cm-1 (2000 spectral points in the LW and 8000 spectral points in the SW channel). It has a spectral resolution of 2 cm-1 and covers a wavelength range from 1.2–45 μm. It is well suited for determining the precise composition of the Martian atmosphere and works by analyzing wavelengths of sunlight in the range of 1.2–45 μm that are absorbed by molecules in the atmosphere and from the infrared radiation they emit.

Table I. PFS parameters
The spectrometer will measure vertical pressure and temperature profiles of carbon dioxide (which makes up 95% of the Martian atmosphere) and seek water, carbon monoxide, methane, and formaldehyde abundances. For the discovery of methane and formaldehyde in the Martian atmosphere, over 2931 spectra were obtained in 24 orbits of the planet focusing on areas located on and near the equatorial region. The highest concentrations of methane were measured over three large areas on Mars known as Arabia Terra, Elysium Planum, and Arcadia-Memnonia. These regions also were associated with high concentrations of hydrogen indicating water, water vapor, or frozen water ice.
Barry E. DiGregorio is a research associate for the Cardiff Centre for Astrobiology (Cardiff, Wales, U.K.).
References
1. Spectroscopy 20(2), 50–51 (2005).
2. Planetary and Space Science 41, 441–451 (1993).
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
