The Use of a Raman Spectral Database of Minerals for the Rapid Verification of Semiprecious Gemstones
The authors discuss the use of Raman spectroscopy to identify an unknown gem or mineral sample or to verify that a known sample has been classified correctly.

Raman spectroscopy is a frequently used analytical technique in the field of geosciences because it can reveal a great deal about the chemical makeup and structure of a mineral (1). Although the Raman spectrum often provides unique information about a sample, it also can be very complex and can be quite difficult to interpret. Even if specific peak assignments are difficult, the Raman spectrum of a gem or mineral can provide a unique fingerprint of the material that can be used to identify an unknown sample or verify that a known sample has been classified correctly.
In many areas of spectroscopy, multivariate statistical analysis techniques have been used to extract useful information from spectral data. One of the simplest and most popular of these techniques is spectral correlation or matching (2). This is basically a vector dot product operation between the spectrum of interest and one or more reference spectra appropriate to the application. For identification purposes, the sample spectrum is compared to a large library of reference spectra and the reference spectra are then ranked according to the correlation value. The reference with the highest correlation value generally will identify the sample if the correct spectrum is in the library. Of course, in the real world, it's not quite this simple. Often, spectral normalization, feature weighting, region selection, and other corrections can be applied before calculating a similarity value. Instead of a simple correlation algorithm, Euclidian, Mahalanobis, or a derivative calculation might be used.
Material verification is a slightly different procedure because you might have significant a priori information about the expected outcome. In this case, instead of asking: "What is this sample?", you are asking: "Is this sample what I expect?" Multiple reference spectra from the same mineral species or group can be used to provide a more reliable verification or perhaps more importantly, determine that there is insufficient information in the spectrum to make a reliable verification. In most situations, "I don't know" is a better response than a misclassified sample (3).
After a good Raman spectrum has been acquired from a sample, the key to the successful application of multivariate statistical analysis techniques is the availability of a reference database of high-quality spectra from the materials that might be encountered in the analysis. Ideally, the database should contain spectra from multiple samples of the same material measured under different sampling conditions. These spectra can provide a statistical basis for determining a confidence value for the material verification. An excellent collection of Raman spectra from minerals has been developed as part of the RRUFF Project at the University of Arizona (Tucson, Arizona) (4–8). This database contains spectra from over 2000 mineral species acquired with different excitation lasers and in some cases, different crystal orientations and samples, and forms the basis for the work reported here.
Many of the minerals frequently encountered as gemstones have unique Raman spectra that can be used to verify that a stone has been identified correctly. Because most gemstones are natural materials created in a very complex environment, spectral variations related to the chemical and structural differences are expected, but the major peaks are quite consistent. In many gemology applications, the goal is to classify samples into three groups: High confidence that the stone is labeled correctly; high confidence that the stone has been labeled incorrectly; and the spectral information is inconclusive and there is a need for further analysis.
One goal of this research was to determine if the high-throughput spectroscopy techniques used in the pharmaceutical industry to screen polymorphs could be applied to gemstones (9). Although it is appropriate to analyze high-value stones such as diamonds, emeralds, rubies, and sapphires individually, there is also a QC requirement for the large volumes of semiprecious stones used in jewelry. Automated sampling techniques would be necessary to rapidly verify that the gemstones in a lot are labeled correctly. For this work, 96 semiprecious gemstones were placed in a glass-bottomed 96 micro well plate normally used in automated analysis of biological samples. Several different approaches were taken to classify the gemstones using multivariate statistical analysis techniques and reference spectra from the RRUFF Raman database.
Experimental
For this study, a small parcel containing several hundred (120 carat total weight) mixed faceted stones was purchased from an Internet site. The semiprecious stones came in multiple colors and different shapes. An important point to make is that the Raman spectra of minerals from the same species are very similar. A mineral species is a group of minerals that have similar chemical composition and crystal structure (11). While the members of a species such as beryl have the same crystal structure and chemical composition (aluminum beryllium silicate), the color and value of the stones can be quite different. Emeralds are very valuable members of this species and have a similar Raman spectrum to aquamarine, another beryl mineral. Natural rubies and sapphires are two of the most valuable gemstones and both are members of the corundum species (aluminum oxide). Natural red corundum generally is classified as ruby and all other colors are classified as sapphire, even though historically, the blue stones were classified as sapphire. Before the advent of synthetic sapphire, pink and orange sapphires were extremely rare and valuable. However, synthetic sapphires also belong to the corundum species and have spectra that are the same as the natural stones even though they are available in a range of colors as well as clear.
Other semiprecious stones in this sample set are quartz. Several members of the quartz species (silicon dioxide) such as citrine (yellow), amethyst (purple), and smoky quartz have been identified: topaz, a fluorine-containing aluminum silicate (Al2SiO4 (F,OH)); zircon (zirconium silicate); and peridot (magnesium iron silicate). Most of the stones were small and fit easily in the 5-mm wells in the sample plate. No attempt was made to center the smaller stones in the wells. Stones were selected from the packet to provide a broad mix of colors and sizes. The order of the stones in the well plate was random and we had no knowledge of the types of stones in the sample set.
Raman Measurements
Raman spectra were acquired from 96 semiprecious gemstones placed in a glass-bottomed micro well plate using a Thermo Scientific DXR Smart Raman system (Madison, Wisconsin). The instrument was configured with the Array Autosampler and used 532-nm laser excitation. The laser power was set to 10 mW and a 50-µm slit was used to reduce data collection times. Four exposures of 5 s each were averaged for each spectrum using a single high-resolution grating (1800 lines/mm), resulting in a spectral range from 50 cm-1 to 1850 cm-1 . The system was configured with an automated x–y stage designed to hold SBS standard micro well plates and was calibrated for the 96 well plate used in this work. The plate was moved to well A1 using the system software (Thermo Scientific OMNIC Array Automation), and the spectral signal was maximized using the Raman system's autofocus capability. The Raman system has an autoexposure feature that automatically determines the exposure time necessary for a desired signal level, but it was not employed in this exploratory work.
RRUFF Raman Spectral Database
Search libraries in the software system were created from subsets of the spectra available on the RRUFF website (10). The RRUFF Project acquired spectra from a number of different Raman configurations. Oriented spectra were acquired with a special system designed for polarization studies using a 514-nm laser; high-resolution spectra were acquired with a Raman instrument (Thermo Scientific Almega) using 532-nm excitation and 780/785-nm excitation. Full-range spectra were also acquired on the Almega instrument for both excitations. The spectral search software allows several libraries to be combined into a single search operation and reports the best results from all of the selected libraries.
Results and Discussion
In the initial study, a single spectrum was acquired from the center of each of the 96 wells. The array automation software creates a colored diagram based upon the acquired spectra. In Figure 1, the color is based upon the total spectral signal, with red indicating the wells with the strongest spectral response. The wells colored purple correspond to very weak or no Raman signal.
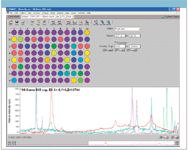
Figure 1: Array automation display for semiprecious gemstones in a 96-well plate.
The bottom portion of Figure 1 shows spectra from various wells in the plate. Clear spectral differences are observed and the remainder of this article will discuss ways to use these spectral differences to classify the stones.
The first technique that we evaluated in this work was spectral searching. Although most spectral search algorithms use some form of vector math to do a point-by-point calculation in the defined spectral range, the precise metrics can weigh the relative peak heights differently. Another important metric called the difference or derivative algorithm takes the difference between two points in the vector before calculating the values (2). This is very helpful in Raman spectroscopy because it significantly reduces the contribution from any broad fluorescence features in the spectrum. If A and B are vectors containing the selected region of two spectra, the match factor coefficient between them is given by the equation:
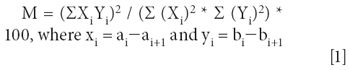
In this example, we selected the spectrum corresponding to well B1 and set up a spectral search using three of the spectral libraries. The results shown in Figure 2 indicate that all of the best matches correspond to samples of topaz analyzed with different laser excitations. A visual examination of the spectrum from well B1 with the top two matches verifies that the spectra are very likely from the same mineral. In this example, the best match was from a spectrum acquired with 780-nm excitation even though the spectra from the well plate were acquired with 532-nm excitation. The fact that spectra acquired with 514-, 532-, and 780-nm lasers all appear in the top matches suggests that the laser excitation did not significantly change the relative peak intensities for this sample. However, for natural minerals from various sources, a match value greater than 90 is a strong indicator that the minerals are the same. For this spectrum, the top seven matches are all over 90 and the next ones also have a good match.
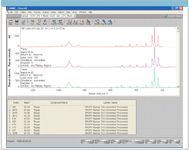
Figure 2: Spectral search with multiple libraries for spectrum in well B1.
Because the match value is calculated using all of the points in a spectral region, it is not obvious how noise affects the result. If we can get adequate results using low signal-to-noise ratio (S/N) spectra, we can greatly reduce the time required to measure multiple stones. We evaluated this by creating a plate image using the integrated area under the strong peak in the quartz spectrum at 460 cm-1 . The quartz group contains a number of members including amethyst and citrine; all of which consist of crystalline silicon dioxide containing trace levels of different metal ions that produce the unique colors. We found the spectrum with the highest value and a second spectrum with a factor of ten less signal. We calculated an S/N by dividing the peak height by the RMS noise in a peak-free region. The S/N for the strong spectrum was over 2000 and the S/N for the weak spectrum was 168. When we did a spectral search of the strong spectrum, the best match value was 97.52, but surprisingly, the match value for the weak spectrum was 94.10. This suggests that in many applications, rapidly acquiring spectra with a S/N of 100 might be sufficient for screening. This is important because reducing the S/N by a factor of ten can reduce the measurement time by a factor of 100. Because of the small size of many of the stones, a single position at the center of the well frequently missed the stone. An important feature of the array automation software is the ability to measure multiple points in a single well automatically. Users have the option of choosing the number of points to analyze and the spacing between the points. In the next example, we chose to measure nine points in the well using a step size of 1000 µm (1 mm). This creates a 3 x 3 grid around the center of the well. Figure 3 shows the results of this measurement on the same samples in the well plate. In this case an intensity value based upon the total Raman signal is calculated for each of the nine points in a well. When a specific well is selected, a magnified version is shown to the right and the corresponding spectra are revealed by clicking on the appropriate position in the expanded well. There are purple points in many of the wells. All but one of the wells has at least one "non-purple" position.
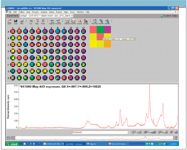
Figure 3: Example of acquiring spectra from multiple points in a well.
One of the objectives of this work was to evaluate Raman spectroscopy as a technique for screening semiprecious gemstones rapidly. So far, we have shown that good Raman spectra can be acquired automatically from samples in a sample plate. In a screening application, the goal is often to verify that the gemstones are classified correctly. A technique similar to Spectral Search can be applied easily to the spectra from the samples in the 96 well plate. We call this technique "Target Verification." If A and B are vectors containing the selected region of two spectra, the cross-correlation coefficient between them is given by the following equation:
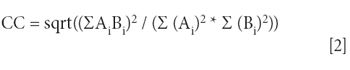
In this example, A is the vector of a high-quality reference spectrum of the mineral that corresponds to a stone that we might encounter (beryl, corundum, garnet, quartz, peridot, topaz, and zircon). We have chosen to verify which of the wells contain garnets by matching the spectra in each well B to a reference spectrum of almandine (Fe3Al2 [SiO4]3 ), which was obtained from the RRUFF website. The garnet group consists of a number of aluminum silicate minerals with a similar crystal structure and a second metal ion in the composition such as iron for almandine. Any points in the well plate with a spectrum that correlates highly with the reference spectrum of almandine are colored red, as shown in Figure 4.
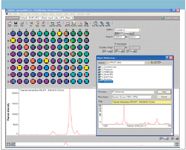
Figure 4: Target verification using garnet/almandine reference spectrum.
This target verification clearly shows which of the samples are garnets. In a rapid verification application, the actual task would be checking for "non-red" wells, which correspond to samples that do not match the reference spectrum. These samples can simply be rejected or set aside for further analysis.
One challenge of any screening technique for minerals is the differences found in the Raman spectra from natural materials. The sample in well D11 is a good example. A target verification with corundum (ruby or sapphire), which is the crystalline form of Al2O3, on the single point map produces a good match for well D11. The stone in well D11 is lightly colored or clear and not red, so it would be classified as a sapphire. When the same verification was applied to the 3 x 3 map shown in Figure 3, one point in the D11 well failed the verification. Two spectra from well D11 are shown in the bottom of Figure 5. The red spectrum matches well to corundum, but the blue spectrum has two very dominant features obviously quite different from corundum.
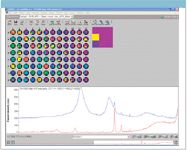
Figure 5: Corundum (red) and unknown spectrum from sample in well D11.
In this case, a simple spectral search of the reference spectra in the RRUFF library with the "bad" spectrum reports several spectra of rutile as the best matches. Although the match value is only 65 due to the presence of the corundum peaks, a visual comparison of the best match confirms that the peaks correspond to the presence of rutile, which is a form of titanium dioxide.
Rutile is a titanium dioxide mineral that can form needle-like inclusion in corundum and is considered acceptable and sometimes desirable in corundum gemstones (11). This example provides further justification for taking the extra time to take multiple spatial measurements on each sample.
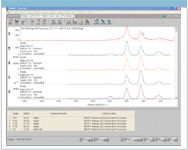
Figure 6: Search results for the inclusion in the corundum sample.
A final area of interest is deciding which wavelength range to use for the analysis. Almost all of the Raman peaks related to gemstones appear below 1800 cm-1 . However, one area of great concern in gem analysis is the verification that an emerald has not been treated with plastic or epoxy to improve its appearance. In this example, a comparison of the sample spectrum and the best match confirm that beryl is a major component of the sample, but several peaks are observed in the spectrum that do not appear in reference spectra from several different beryl samples.
Emerald is the most valuable member of the beryl group, which consists of minerals with the chemical composition Al2Be3Si6O18. Emeralds are rather brittle and often contain cracks that may strongly affect the appearance of the gemstone. The appearance of the stone can be improved greatly by filling the cracks with a polymer of epoxy with a similar index of refraction. Identifying treated stones can be quite difficult when only using the spectral region from 1200 to 50 cm-1 as shown in Figure 7.
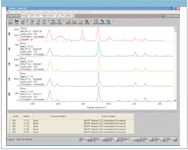
Figure 7: Search results for narrow range spectrum of contaminated beryl.
Most organic chemicals, plastics, and epoxies can be detected easily using a full range spectrum (3500–50 cm-1 ). A second spectrum of the beryl sample was obtained from the RRUFF database that clearly reveals peaks around 3000 cm-1 that correspond to the organic C–H modes. The presence of a plastic can be verified easily by comparing the full range spectrum of the sample to the Aldrich Raman spectral library. The Aldrich Raman library consists of over 14,000 spectra acquired on a Thermo Scientific Nicolet FT-Raman instrument from commercial chemicals and polymers (12). Even though the Aldrich spectral database was acquired on an FT-Raman system, which can change the relative intensities, the best matches clearly indicate that an acrylic polymer is present in the beryl sample.
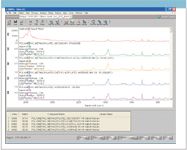
Figure 8: Search results for Aldrich library.
Although the plastic contamination was due to the sample-mounting process for this sample, similar spectral features are found commonly in the plastics and epoxies used to treat gemstones. The specificity of Raman spectra also can be used to verify that the organic material is not a natural oil or wax whose presence is considered acceptable.
Conclusions
A modern Raman instrument designed for routine analysis can be configured with an automated sampling system to rapidly acquire spectra from 96 samples. The fact that different samples produced different levels of Raman signal suggests that the autoexposure feature should be used to obtain a desired signal level. The combination of such an instrument with a large database of high-quality Raman spectra acquired from a large number of mineral samples can be used to verify that semiprecious gemstones are labeled correctly. The use of spectra from multiple samples acquired with different laser excitations greatly increases the reliability of the methods and may be used to determine "confidence" in the results. Although most mineral samples produce Raman spectra in the range of 1800–50 cm-1 , the use of the "Full Range" option on the Raman instrument provides a valuable tool for verifying if a sample has been treated with polymer or epoxy.
Steve Lowry, Dick Wieboldt, and Dave Dalrymple are with Thermo Fisher Scientific, Madison, Wisconsin. Renata Jasinevicius and Robert T. Downs are with Geosciences Department, University of Arizona, Tucson, Arizona.
References
(1) S. Bernard, O. Beyssac, and K. Benzerara, Appl. Spectrosc. 62(11), 1180–1197 (2008).
(2) S.R. Lowry , Automated Spectral Searching in IR, Raman and NIR Spectroscopy, Handbook of Vibrational Spectroscopy, Vol. 3 1948–1961 (2002).
(3) Personal communication with Michael M. Scott, President, White Rose Enterprises, Los Altos, California.
(4) M.B. Denton, R. Sperline, J. Giles, D. Gilmore, C. Pommier, and R.T. Downs, Aust. J. Chem. 56, 117–131 (2003).
(5) R.T. Downs, The RRUFF Project: an integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. Program and Abstracts of the 19th General Meeting of the International Mineralogical Association in Kobe, Japan. O03–13 (2006).
(6) M. Denton, Proceedings of the 4th GIA Research Conference, Gems & Gemology 42(3), 89 (2006).
(7) R.T. Downs, Proceedings of the 4th GIA Research Conference, Gems & Gemology 42(3), 89 (2006).
(8) S. Lowry and J. Workman, Proceedings of the 4th GIA Research Conference, Gems & Gemology 42(3), 91 (2006)
(9) S. Lowry, D. Dalrymple, A. Song, V. Rosso, C. Pommier, and J. Venit, J. Assoc. Lab. Automation 11(2), 75–84 (2006).
(10) RRUFF Website: http://www.rruff.info
(11) W. Schumann, Gemstones of the World (Sterling Publishing Co., New York, January 1, 2007), 3rd Rev Exp edition.
(12) Aldrich Raman Condensed Phase Library for OMNICTM, Thermo Fisher Scientific part number 834-004001.

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.