The Use of X-Ray Powder Diffraction (XRD) and Vibrational Spectroscopic Techniques in the Analysis of Suspect Pharmaceutical Products
Spectroscopy
Here, we compare XRD and FT-IR for analysis of suspect counterfeit pharmaceuticals to determine how the techniques can be used in a complementary fashion.
As the global marketplace for pharmaceutical products increases, so does the potential for counterfeit products entering the legal distribution supply chain, posing a major threat to public health. Counterfeit pharmaceuticals are unregulated products that may contain the incorrect amount of active pharmaceutical ingredient (API), a different API, no API, or incorrect excipients within the counterfeit product. Types of pharmaceutical products counterfeited include solid dosage forms (tablets and capsules) and injectable products (such as oncology drugs). Historically, counterfeit products have ranged from poor to very sophisticated copies of the original product. Depending on the type of product encountered, a variety of different physical and instrumental analysis techniques may be required to distinguish between a suspect counterfeit and authentic product. It is important to use multidisciplinary approaches when analyzing suspect counterfeit products. The Food and Drug Administration (FDA)’s Forensic Chemistry Center continually investigates new instrumentation and methods to add to the growing list of instrumental techniques available to analyze suspect counterfeit products.
Counterfeit pharmaceutical products pose a major threat to public health. Counterfeit pharmaceuticals are unregulated products which may contain dangerous, harmful ingredients, or insufficient amount of the active pharmaceutical ingredients (APIs). These illegally manufactured products have been found widely distributed throughout the world, which presents an ongoing public health threat (1–4). There are many different counterfeit products (4–7), requiring multiple analytical techniques to authenticate (4,8–10). X-ray powder diffraction (XRD) is a technique used to analyze various types of trace evidence (glass, paint, and drugs [11,12]). Previous work has demonstrated that X-ray diffraction is useful in pharmaceutical analysis, including the analysis of suspect counterfeit products (13–15). Combined with Fourier transform infrared (FT-IR) spectroscopy, XRD can be used to differentiate suspect counterfeit products from authentic products.
Estimates vary, but the highest percentages of counterfeit products are often found in developing countries. Examples of counterfeit pharmaceuticals include brand name and generic products in a wide variety of therapeutic groups such as antibiotics, antihistamines, anti-malarials, hormones, steroids, lifestyle drugs, and other products. Patients with prolonged or chronic illnesses may experience exacerbated symptoms or death as a result from taking a counterfeit medication. For example, if preventative medications such as anti-malarial pharmaceuticals are counterfeit, the patients would still be at risk of developing and transmitting the disease.
The Food and Drug Administration (FDA)’s Forensic Chemistry Center (FCC) has been involved in the analysis of suspect pharmaceutical products for more than 20 years. This has allowed the FCC to develop extensive expertise in the forensic examination of pharmaceutical APIs and dosage forms. A multidisciplinary approach incorporates a broad array of laboratory instrumentation, methods, and expertise. Depending on the type of product encountered, a variety of different physical and instrumental techniques may be required to distinguish between a suspect counterfeit and the authentic product. These include visual or optical microscopic analysis, molecular spectroscopy, elemental analysis, and a variety of separation techniques (8,9). Many of these laboratory techniques and methods are now being used to analyze pharmaceutical products outside of the laboratory setting (9).
XRD is a technique which has found use in forensic science (11,12). Often, XRD is used for phase identification of crystalline materials, and to obtain unit cell dimensional information of materials (16,17). Most materials have distinct X-ray diffraction patterns, and can be identified within a compound or mixture when compared to databases of known XRD patterns. The use of XRD for the identification of pharmaceuticals has been demonstrated in various studies (3,14,15). Several of these studies have shown that XRD can be used to determine the presence of API and other materials with limited sample preparation of the dosage form. One study obtained XRD patterns of intact tablets after the removal of the tablet coating, while other studies were able to obtain XRD patterns of dosage forms through the blister packaging.
This paper describes an XRD method and its comparison to FT-IR spectroscopic methods used in the analysis of suspect counterfeit pharmaceutical products. The goal was to determine the advantages and limitations of XRD and determine what complementary information XRD could provide to the FT-IR analysis of suspect counterfeit dosage forms.
Experimental
A Bruker AXS D2 Phaser X-ray diffractometer configured with a Cu-Kα source and a LynxEye Detector was used to analyze all samples for this study. The data acquisition parameters included a scan range of 19 ° to 135 ° 2Theta step size of 0.03 °, and step time of 0.3 sec. The total run time for each sample was 20 min. The Bruker AXS Measurement Suite and Diffrac. EVA V2 software packages were used to collect and evaluate the diffraction patterns of samples collected. A daily performance check of the instrument was performed using an aluminum oxide (Al2O3) reference sample provided by Bruker.
The FT-IR spectra of all tablets (authentic and suspect), APIs, and excipients had been collected previously using a Thermo Nicolet 8700 FT-IR spectrometer with a DTGS detector. The sample preparation method used is described elsewhere (8,9). Each FT-IR measurement was made using a SensIR Durascope attenuated total reflection (ATR) attachment. The single reflection internal reflectance element (IRE) consisted of diamond coated zinc selenide (ZnSe). Each FT-IR spectrum was collected using 4 cm-1 resolution, 64 coadditions over a spectral range of 4000 to 650 cm-1. Omnic (version 8.0) was used to evaluate the FT-IR spectra, and apply an ATR correction to each FT-IR spectrum.
A series of authentic products, APIs and excipients were identified as candidates to generate the initial XRD library (Table I). The criteria used for authentic product selection included products representing a variety of different therapeutic categories, a range of different API concentrations, and products known to be counterfeited. Based on the ingredients listed for each authentic product, product specific APIs and excipients were analyzed by XRD to establish a library of standard XRD spectra for comparison to the authentic and suspect counterfeit dosage forms.
XRD API and Excipient Standards Preparation
Different preparation methods were tested, which included varying the grinding method (by hand or mechanical), amount of powder used, grinding time, and compression pressure. XRD diffraction patterns were collected from each preparation and the data collected reviewed. Based on the XRD patterns collected, the best method involved grinding the powder (excipient or API) and compressing it into a pellet using a Carver Press (International Crystal Laboratories). The excipient or API (120–200 mg) powder was placed into a polystyrene vial (Sigma Aldrich) with a plexiglass grinding ball (Sigma Aldrich). The powder was ground using a Wiggle Bug (Cresent) for 10 s, and, after grinding, 60 mg of ground powder was placed into a 1 cm diameter pellet die (International Crystal Laboratories), and compressed at 4,000 psi using a Carver press. Each pellet was placed on a poly(methyl methacrylate) (PMMA) (Bruker) sample holder, mounted into the XRD sample compartment, and an X-ray diffraction pattern was collected for the sample. The pellet was placed into a 20 mL glass vial for storage.
XRD Dosage Form Sample Preparation
As with the APIs and excipients, multiple preparation methods were tested, including analyzing the whole tablet (intact), removing the tablet coating then analyzing the tablet, and using the same preparation method used for the excipients and APIs. The minimal sample preparation methods (intact tablet, removing tablet coating) did not provide acceptable XRD patterns that could be used for comparison to the standard materials. It was found that the same sample preparation method used for the excipients and APIs provided the optimum XRD patterns for each dosage form.
A tablet (coating and core) was cut into pieces, and half of the tablet placed into a polystyrene vial with a polystyrene grinding ball. The tablet was ground, and the powder was placed into a 1 cm diameter pellet die. The ground tablet was compressed at the same pressure as the excipients and APIs using a Carver press. The tablet pellet was placed on a PMMA sample holder, an X-ray diffraction pattern was collected, and the pellet was placed into a 20 mL glass vial for storage.
Discussion
XRD Analysis of Products
To determine if the powder or tablet preparation method changed the material’s crystalline form, two different crystalline (polymorphic) forms of titanium oxide (TiO2) were prepared and analyzed using XRD. The X-ray diffraction patterns collected of the two different forms of TiO2, rutile and anatase, were compared to the X-ray powder diffraction patterns found in the literature (18). The XRD patterns measured were consistent with the literature, indicating the sample preparation method used did not alter the crystalline form of the materials or add any artifacts to the diffraction patterns.
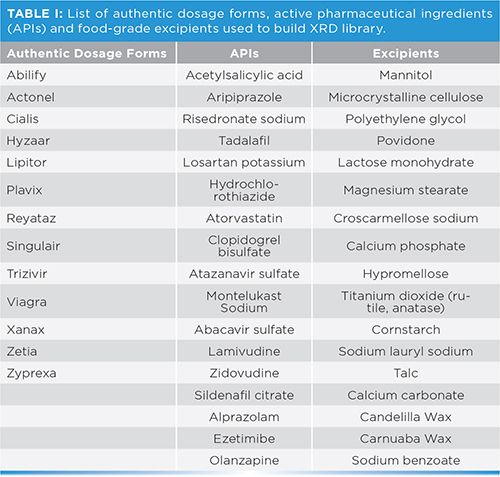
Examples of the XRD diffraction patterns collected of three excipients, and their associated FT-IR spectra are shown in Figure 1. The diffraction patterns of talc and lactose monohydrate exhibit sharp peaks with a high signal-to-noise ratio (S/N) compared to the diffraction pattern of microcrystalline cellulose (MC) (Figure 1). The XRD pattern of MC exhibits broad bands with low a S/N, indicating MC is more amorphous when compared to talc and lactose monohydrate. The FT-IR spectra of all three excipients exhibit strong infrared (IR) peaks over the entire spectral range. In tablet formulations, most of the tablet core formulation consists of a mixture of excipients at varying concentrations and determining the presence or absence of individual excipients in the FT-IR spectra may be complex, due to strong overlapping bands. However, the XRD patterns of excipients appear to be more selective (for example, sharper bands) and therefore may be more useful in trying to confirm the absence or presence of a specific excipient material.
Figure 1: Comparison of XRD patterns and FT-IR spectra of three common pharmaceutical excipients: (1a, 1d) talc, (1b, 1e) lactose monohydrate, and (1c, 1f) microcrystalline cellulose. For (a), (b), and (c), x- and y-axes are 2Theta (WL = 1.54060) and Counts, respectively. For (d), (e), and (f), x- and y-axes are Wavenumbers (cm-1) and Absorbance, respectively.
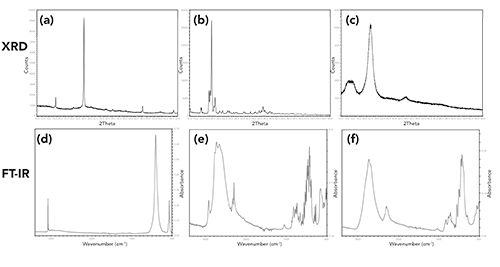
XRD patterns were collected for all the authentic products, and Figure 2 shows the XRD patterns of four different authentic products. When compared to each other, the XRD patterns for authentic products 1, 2, and 4 are unique, and exhibit numerous peaks that can be used for comparison to suspect products. However, several authentic products analyzed (Table I) exhibited the same or similar XRD patterns. An example of this is shown in Figure 2. The XRD pattern of authentic Product 3 is consistent with the XRD pattern of Product 2, indicating the products have similar product formulations. This was confirmed by reviewing the ingredients list and the FT-IR spectra of Products 2 and 3. Products 2 and 3 contain different APIs, but at very low amounts, with the remainder of each product formulation being the similar.
Figure 2: (a through d) Examples of X-ray powder diffraction patterns of four different authentic dosage forms.
The XRD patterns of the other authentic dosage forms were then compared to their respective API and excipient XRD patterns. The XRD pattern of the API and excipients known to be present in each authentic product were overlaid and visually compared to determine if unique peaks could be used to indicate the presence or absence of the various materials. In most cases, the XRD patterns of the authentic products were dominated by the highest concentration excipients in the formulation. These results are like FT-IR, where the IR spectrum of the tablet core is dominated by the higher concentration API(s) or excipients.
It was difficult to visually determine the presence of the API within the authentic dosage form XRD patterns. This may be due in part to the manipulation of the API during the manufacturing process (granulation, blending, and tablet compression). Also, the concentration of the API in each of the authentic products (weight percent) may be below the detection limit for XRD and the method. Therefore, the presence or absence of APIs could not be used as a means for determining authenticity of a product using XRD spectral analysis.
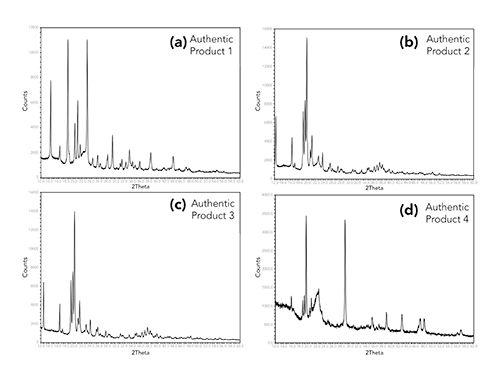
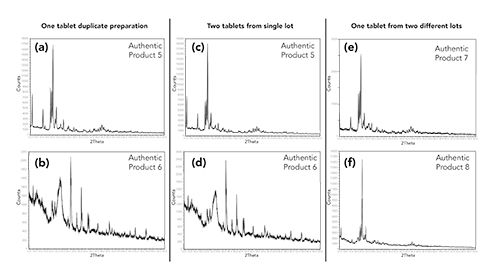
Because of this, it was decided to use the overall XRD pattern (profile) for comparison of suspect counterfeit products to authentic products. The United States Pharmacopoeia (USP) general chapter <941> states that a variance in peak positioning (peak shifts) of 0.2° 2Theta or less are to be expected (19). This is due in part to the orientation of the material in the pellet and the slight changes in crystallinity of the material. Variances greater than 0.2° 2Theta would be indicative of a different crystalline structure of a material (17). Using authentic products, experiments were conducted to determine the variability of duplicate measurements of a single tablet, variability between tablets from the same lot, and lot-to-lot variability. The XRD patterns were collected, and individual peak positions were measured for each. Examples of these experiments are shown in Figure 3. In all cases, peak shifts observed were less than 0.2° 2Theta. Based on the results of the comparison of the authentic dosage form XRD patterns to their associated APIs and excipients and the reproducibility and variability experiments, the criteria for comparing a suspect counterfeit product to an authentic product were established. If the XRD pattern of the suspect product has additional peaks, missing peaks, or if the peak positions were shifted greater than 0.2° 2Theta compared to an authentic product, the suspect product would be considered not consistent with the authentic.
Figure 3: X-ray powder diffraction patterns of four different authentic products to assess reproducibility and variability. (a) and (b) Two pellets (red vs. black) created from the same tablet. (c) and (d) Two pellets created from two tablets (red vs. black) from the same lot number. (e) and (f) Two pellets (red vs. black) created from two tablets from two different lot numbers.
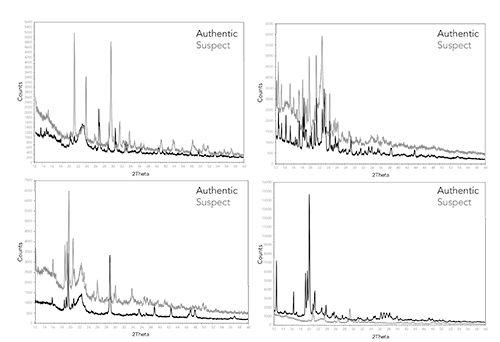
A series of suspect products previously analyzed using FT-IR were prepared as above and analyzed using XRD. XRD patterns were obtained and compared to their respective authentic products. Examples of these comparisons are shown in Figure 4. In all cases, the suspect products exhibit very different XRD patterns when compared to the authentic products, demonstrating that the XRD method works well as an authenticity screening method and is useful in confirming the results obtained by FT-IR analysis. Based on the FT-IR analysis, the suspect products had very different formulations when compared to the authentic product.
Figure 4: X-ray powder diffraction spectra of four different suspect counterfeit products and their associated authentic pharmaceutical products.
Comparison of XRD to FT-IR
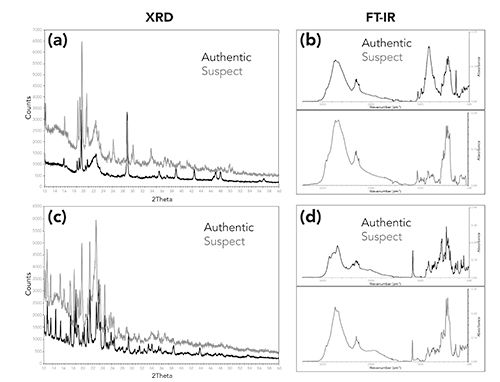
Both XRD and FT-IR can generate spectral profiles of authentic products, which can be used for comparison to suspect counterfeit products. These authentic profiles can be used to analyze suspect counterfeit products rapidly. Figure 5 shows two examples of XRD patterns and FT-IR spectra obtained for the same set of products. In both examples, the suspect products can be distinguished from their respective authentic products. However, determining the formulation differences between the suspect and authentic products is easier with FT-IR than with XRD. With FT-IR, determining individual formulation components can be accomplished using spectral searching, data processing techniques (such as spectral subtraction) and spectral interpretation. However, in this example the XRD patterns identifying individual peaks or groups of peaks associated with formulation ingredients in the XRD patterns can be challenging.
Figure 5: Comparison of the X-ray powder diffraction pattern and FT-IR spectra of two different suspect counterfeit products and their associated authentic products. (a, b) Product 1, and (c, d) Product 2.
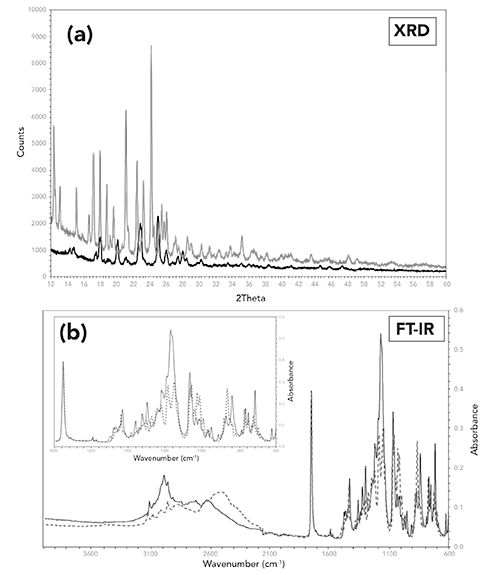
The crystallinity of an API will determine its solubility. In some cases, an API may have two or more different crystalline or polymorphic forms, with each crystalline form having a different solubility. These differences in solubility may either increase or decrease the bioavailability of the API. Figure 6 is an example of an API which exists as two different polymorphic forms (1 and 2). Both XRD and FT-IR can differentiate between the forms; however, the XRD patterns are more distinct (sharper peaks, less overlap) compared to the FT-IR spectra. Although the XRD patterns are easily differentiated in the bulk API, being able to identify the different forms in a tablet formulation may be problematic. This is based on the experience of trying to visually identify unique peaks associated with the API in the XRD pattern of a tablet formulation.
Figure 6: Comparison of (a) the X-ray powder diffraction patterns, and (b) FT-IR spectra of two different polymorphic forms of the same API (Form 1 (black) and Form 2 (red). An expanded view of the IR fingerprint region is shown in Figure 6b.
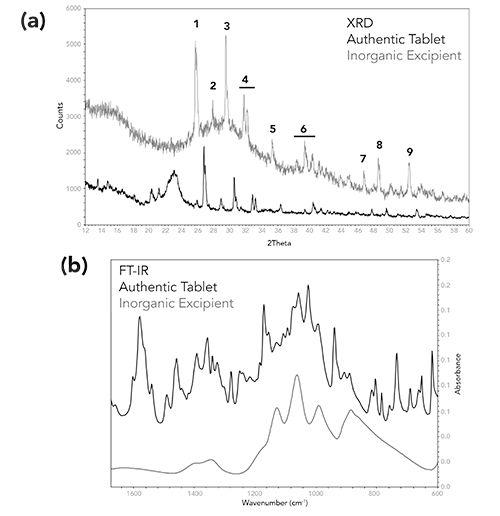
For most excipients and APIs analyzed, the XRD patterns exhibited sharp peaks especially for inorganic excipient materials (such as silicates, carbonates). Some tablet formulations use inorganic excipients, and the amounts used are product-dependent. The presence or absence of inorganic excipients may be useful in determining the authenticity of a suspect product, especially in cases where the suspect is a sophisticated copy, and very small FT-IR spectral differences are observed between the suspect product and the authentic. Figure 7 is an example of an authentic product which contains a low amount of an inorganic excipient. The FT-IR spectrum of the authentic tablet core is dominated by the API and the higher concentration excipients. The lower concentration inorganic excipient is strongly overlapped making it impossible to determine if this material is present in the formulation. Comparing the XRD pattern of the authentic tablet to the XRD pattern of the inorganic excipient, the peaks associated with the inorganic excipient are easily observed. Although the peaks of the standard are shifted compared to the tablet, the number of peaks and the peak height ratios are consistent between the standard and the tablet. The peak shifts between the standard and tablet are likely due to the manipulation of the inorganic excipient material during the manufacturing process.
Figure 7: Comparison of (a) the X-ray powder diffraction pattern, and (b) FT-IR spectra of an authentic product (black) and a low concentration excipient ingredient (red). (a) The peaks used for comparison in the XRD pattern are numbered #1 through #9.
Conclusions and Future Work
A method was developed to analyze and produce quality XRD spectra of authentic pharmaceutical products, APIs, excipients, and samples of suspected counterfeit pharmaceutical products. Criteria were established to determine the authenticity of a pharmaceutical product based on comparing the XRD pattern profiles of a suspect to an authentic product. A limited amount of formulation information can be obtained from the XRD pattern profiles of tablets; however, identifying specific peaks associated with the API is difficult. This type of non-destructive, minimal sample preparation technique could be useful when analyzing large numbers of pharmaceuticals.
XRD is a complementary technique to FT-IR spectroscopy. Both techniques generate information-rich spectra that can be used for determining authenticity of pharmaceutical products. Combining these techniques in the analysis of a suspect counterfeit product provides a more complete view of the sample which can be useful in the analysis of sophisticated products. Both XRD and FT-IR are solid state analytical techniques allowing for the sample to be retained for further analysis using other laboratory instrumental methods. Currently, spectra are overlaid and visually compared to determine authenticity; however; automating the spectral comparison by incorporating spectral searching capabilities would be useful. Finally, it would be beneficial to modify the current sample preparation method or develop a new method to better identify the APIs within the dosage forms.
Acknowledgments
The author thanks JaCinta Batson and Dr. Cheryl Flurer for reviewing this paper and Dr. Daryl Mincy for his initial feasibility work in collecting XRD data of authentic products and useful discussions.
Disclosure
The opinions in this paper are the authors and should not be considered opinions or policy of the U.S. Food & Drug Administration. The mention of specific products and instruments in this paper is for information purposes only and does not constitute an endorsement by the Food and Drug Administration or the Forensic Chemistry Center.
References
- A.K. Deisingh, Analyst 130, 271 (2005).
- T.K. Mackey, B.A. Lang, P. York, and T. Kubic, Am. J. Trop. Med. Hyg. 92(s6), 59 (2015).
- A. Koczwara and J. Dressmen, J. Pharm Sci. 106, 2921 (2017).
- H. Rebiere, P. Guinot, D. Chauvey, and C. Brenier, J. Pharm. Biomed. Anal. 142, 286 (2017).
- T.K. Mackey, R. Cuomo, C. Guerra, and B.A. Liang, Nat. Rev. Clin. Oncol.12(5), 302 (2015).
- Counterfeit Medicine, https://www.fda.gov/drugs/buying-using-medicine-safely/counterfeit-medicine (accessed 02/18/2020)
- FDA Press Release, April 13, 2018: “Canadian Drug Firm Admits Selling Counterfeit and Misbranded Prescription Drugs Throughout the United States,” https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/criminal-investigations/april-13-2018-canadian-drug-firm-admits-selling-counterfeit-and-misbranded-prescription-drugs (accessed 02/18/2020)
- D. Bansal, S. Malla , K. Gudala, and P. Tirari, Sci. Pharm. 81(1), 1–14 (2013).
- A. Lanzarotta, K. Lakes, C.A. Marcott, M.R.Witkowski, and A.J. Sommer, Anal. Chem. 83, 5972 (2011).
- A. Lanzarotta, N. Ranieri, D.A. Albright, M.R. Witkowski, J.S. Batson, and M. Fulcher, Am. Pharm. Rev. 18(3), 24 (2015).
- P. Thatcher and G. Briner, Powder Diffr. 1(4), 320 (1986).
- M. Kotrly, Z. Kristallogr. Suppl. 23, 35 (2006).
- J.K. Maurin, F. PluciÅski, A.P. Mazurek, and Z. FijaÅek, J. Pharm. Biomed. Anal. 43(4), 1514 (2007).
- I. Jendrzejewska, P. Zajdel, E. Pietrasik, Z. Barsova, and T. Goryczka, Monatshefte Fur Chemie 149, 977 (2018).
- M. R. Caira, Am. Pharm. Rev. 17(1), 54 (2014).
- A.A., Bunaciu, E. Udristioiu, and H.Y. Aboul-Enein, Crit. Rev. Anal. Chem. 45(4), 289 (2015).
- B. Dutrow and C.M. Clark, “Geochemical Instrumentation and Analysis: X-ray Powder Diffraction (XRD).” https://serc.carleton.edu/research_education/geochemsheets/techniques/XRD.htmL (accessed 02/18/2020)
- K. Thamaphat, P. Limsuwan, and B. Ngotawomchai, Kasetsart J.: Nat. Sci. 42(5), 357 (2008).
- USP Pharmacopeial Convention. General. <941> Characterization of Crystalline and Partially Crystalline Solids by X-ray Powder Diffraction (XRPD) USP 42-NF37 S2, 7097 (2019).
Mark R. Witkowski is a chemist in the Trace Examination Section at the U.S. Food and Drug Administration’s Forensic Chemistry Center, in Cincinnati, Ohio. Kelsey DeWitt is a forensic chemist in the drug chemistry unit at the Raleigh/Wake City-County Bureau of Identification, in Raleigh, North Carolina. Direct correspondence to: Mark.Witkowski@fda.hhs.gov

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.