Using SR-IMS to Study the Fate and Transport of Organic Contaminants in Plants
The fate and transport of organic contaminants and their impact upon plant development has been an important topic in environmental science. Here the authors report the use of synchrotron radiation Fourier-transform infrared microspectroscopy (SR-IMS) as a direct method for monitoring the fate and effects of 2,6-dinitrotoluene (2,6-DNT) in maize (Zea mays L.) root tissue.
Over the past decade, Fourier-transform infrared microspectroscopy (IMS) has emerged as a tool to study plant growth and development at the molecular level. Synchrotron radiation (SR) as a source for IMS has offered improvements in studying plant samples with limited light throughput. The small effective source size and high brightness of the synchrotron beam also allows study of samples with small areas with spatial resolution to the diffraction limit of 5–15 μm. However, the use of SR-IMS to study plant structures is still in its infancy. There are a limited number of research groups that use IMS to study plant microstructures with McCann's (1, 2) group at Purdue, and Schreiber et al. (3, 4) at Universität Würzburg in Germany, leading the way. Only a few scientists have used synchrotron facilities for this type of research. Raab et al. (5, 6) studied biological and chemical changes in growing legume roots at the Advanced Light Source (ALS) of Lawrence Berkeley National Laboratory, Berkeley, California. Wetzel et al. (7) and Yu et al. (8, 9) studied the microstructures of wheat and barley seeds, respectively, at the National Synchrotron Light Source (NSLS) of Brookhaven National Laboratory, Upton, NY. The results of these studies suggest a potential use of SR-IMS to analyze changes in plant structures due to contact with organic contaminants, commonly called herbicides.
The main focus of our research is studying the fate and transport of organic contaminants in plants. Phytoremediation, the use of plants to remove contaminants from water, soil, or air, has grown greatly over the past 20 years. Nevertheless, an effective technique to determine the fate of organic contaminants directly in plants is needed. Typical extraction methods degrade plant tissues or must be applied to ground-up tissue, while most radiolabel techniques cannot pinpoint the anatomical region in which the contaminants are ultimately bound or transformed. SR-IMS provides a direct alternative to these techniques, combining spatially localized information and chemical information from the IR absorbances to produce a chemical map that can be linked to a particular morphology or functional group. For herbicides, the tolerable levels can to be too low for direct detection, but the effects of the compound on plant structures might be of interest. In the case of many common contaminants, a several hundredfold concentration of the contaminant accompanies its uptake into a plant with the transpiration stream.
Currently, our lab is using SR-IMS to study the fate and transport and developmental effects of common aromatic and heterocyclic organic contaminants in sunflower (Helianthus annuus) and maize (Zea mays) plants. Here we will report on the fate and effects of 2,6-dinitrotoluene (2,6-DNT), an industrial byproduct of TNT production, on maize plants.
Materials and Methods
Sample Preparation. Pioneer Maize seeds (hybrid 32M38) were germinated for three days in vermiculite moistened with sterilized tap water and then transferred to 50-mL centrifuge tubes containing full-strength Hoagland's solution for a 24-h period. Hoagland's solution was prepared as previously reported by Castro et al. (10). The maize seedlings then were distributed into stratified groups with each group having a similar mean weight. The seedlings were grown hydroponically in full-strength Hoagland's solution containing 0, 5, 10, and 15 mg/L 2,6-DNT for a one-week period under continuous lighting. Three plants were used for each treatment group. After the growth period, the maize plants were removed from their treatment solutions and rinsed briefly with deionized water before dissection and cryomicrotome sectioning. The remaining treatment solutions were kept for analysis by high performance liquid chromatography (HPLC). The representative secondary roots from each treatment were pooled (~6 roots/treatment) and samples were cut into 1-cm segments from the tip. The root tissues were frozen onto specimen blocks surrounded by Tissue-Tek OCT (Sakura Finetek USA, Inc., Torrance, CA) at –40 °C, in preparation for sectioning at –20 °C. The 4-μm thick frozen sections were thaw mounted onto infrared-reflecting "E" glass microscope slides (Smiths Detection, Danbury, CT). The slides then were stored at room temperature until analysis.
Monitoring of 2,6-DNT uptake. The amount of solution used by the seedlings was calculated, while measuring contaminant uptake by the maize plants was performed using an Altex liquid chromatograph Model 330 attached to a spectrophotometer flow cell (Altex/Hitachi model 100-40) set up for detection at 275 nm. The separation was through a Hamilton (Reno, NV) PRP-1 column (150 × 4.1 mm) with an eluent consisting of methanol:water (9:1) at a flow rate of 1.0 mL/min. Loss of 2,6-DNT from the residual treatment solutions was observed, with 100%, 53%, and 16% depletion at the 5, 10, and 15 mg/L concentrations, showing degradation and uptake by the maize. Water uptake varied with treatment.
Synchrotron radiation Fourier-transform infrared microspectroscopy (SR-IMS). SR-IMS was conducted at the NSLS at Brookhaven National Laboratory at beamline U2B using a Magna 860 spectrometer (Thermo Nicolet Instruments, Madison, WI) coupled with a Nicolet NicPlan IR microscope with a custom-made external cryostat chamber. An in-depth description of Beamline U2B along with a schematic can be found in a report authored by Marinkovic et al (11). The narrow-range, internal mercury cadmium telluride (MCT/A) detector was used, covering a range of 4000–650 cm-1. All spectra were collected using the 32× IR objective (Thermo-Spectra-Tech, Shelton, CT) in the reflection mode. The reference spectrum was collected from an unused portion of the low "E" slide at 128 scans with 4-cm-1 resolution. The spectra were recorded in absorption mode at a resolution of 4 cm-1 with 64 scans co-added to reduce signal-to-noise ratio (S/N). A 30-μm square aperture was used for scouting potential specimens for mapping and taking individual spectra. A 12-μm square aperture with 10-μm steps was used for generating an IR map of root sections using the Atlμs, a component of the Omnic 6.0 software package (Thermo Electron, Madison, WI).
These spectra are diffraction limited; the plant contains interesting smaller features.
Results and Discussion
Radiolabeling of organic contaminants is the method of choice for studying the fate and transport of organic contaminants in plant tissues for most environmental scientists. However, many compounds are not available in isotopically labeled form, and the microstructures of the plant are destroyed during the extraction processes used to determine the location of radioactivity. This limits determining the spatial location and distribution of the organic contaminants. The chemical imaging ability of SR-IMS provides a direct method of pinpointing which plant structures are changed due to exposure and the anatomical region where the organic compound ultimately can be bound or transformed.
The chemical image produced from SR-IMS uses different colors to represent chemical group intensities generating a false color intensity map. Figure 1b shows the distribution of nitro compounds in root tissue of a maize plant treated with 10 mg/L 2,6-DNT by taking the ratio of peak heights at 1550 (amide II band) and 1530 cm-1. The mapped area covered a region of 290 × 70 μm or 29 × 7 pixels. Raw spectra from the vascular region of 10 mg/L 2,6-DNT treated root tissue in Figure 1e shows the peaks used to generate the peak height ratio map. Blue and green colors signify high intensities, while red and white show little or no presence of the nitro groups. The intensity range extends from 0 to 175 (Figure 1c), whereas a comparable control map would have a range of 0–0.04 without any distinguishing features. The epidermis and vascular region of the root tissue display the highest distribution of nitro groups compared to little or no distribution in the cortex. This provides evidence that 2,6-DNT may be incorporated into the lignin portion of the plant because lignin is predominantly found in the epidermis and vascular region of the root. Similar results have been observed in studies of sunflower plants treated with 1H-benzotriazole (13).
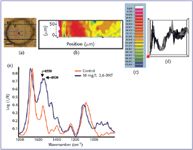
Figure 1. False color intensity map of ratio of peak heights at 1550 and 1530 cm-1, showing the distribution of nitro groups in 2,6-DNT treated maize root tissue at each pixel. (a) Visual image of maize root cross section approximately 500 μm from the tip. The blue rectangles in the visual images represent the mapped area of 29 3 7 pixels. The black bars represent a length of 100 μm. e = epidermis, c = cortex, v = vascular region; (b) chemical image; (c) chemical intensity ruler; (d) 3-D image; and (e) raw spectra of untreated and 10 mg/L 2,6-DNT treated root vascular tissue.
Spectra taken of the cortex of untreated, 5 mg/L 2,6-DNT, and 10 mg/L 2,6-DNT treated maize root tissue were very similar although there are important differences in ratios of several peaks (Figure 2b). Spectra from 15 mg/L 2,6-DNT treated root tissue are not shown because the root tissues were dark and badly damaged, making it difficult to obtain quality spectra from these samples. There does not appear to be a large decrease in the protein (1550 and 1650 cm-1) or carbohydrate (900–1100 cm-1) regions of the treated tissue, and there are no strong bands indicative of the presence of 2,6-DNT. The dominance of the nitro group peaks at 1530 cm-1 and 1350 cm-1 can be seen in Figure 2a, which shows the spectrum of 2,6-DNT. The presence of the 1530 cm-1 peak can be seen in the typical spectra of vascular tissue from maize plants treated with 2,6-DNT (Figure 2c) as a large shoulder on the 1550 cm-1 amide II peak. The obvious overall increase in absorption over the whole 1500–1600 cm-1 region but not in the carbohydrate region (1200–1000 cm-1) does suggest that the changes are due to treatment with 2,6-DNT and not due changes in section thickness or denser tissue. In some cases, the 1530 cm-1 peak might be higher in absorption than the amide II peak at 1550 cm-1, as seen in Figure 1e. Bands due to the aromatic ring of 2,6-DNT appear in the treated samples at 820–975 cm-1. The peak at 820 cm-1 is indicative of C–C ring stretch followed by a ring H–C–C bend near 842 cm-1. Peaks due to the methyl group of 2,6-DNT are at 895 cm-1 (CH3 rock) and 945 cm-1. Decreases in the carbohydrate region and changing ratio of protein bands indicate that the uptake of 2,6-DNT by the plant might have altered their production. Detailed interpretation of these spectral changes requires use of principal components analysis or similar discriminating technique (14 ).

Figure 2. (a) Spectrum of 2,6-DNT prepared in a 1% (w/w) pellet of KBr; (b) typical spectra of cortex of root tissue from untreated, 5 and 10 mg/L 2,6-DNT treated maize plants; (c) typical spectra of vascular region of root tissue from untreated, 5 and 10 mg/L 2,6-DNT treated maize plants. Each example spectrum is an average of 20 spectra from their corresponding region.
Conclusions
SR-IMS was used to determine the distribution of 2,6-DNT in maize root tissue. Chemical imaging of the root tissue showed high levels of the compound in epidermis and vascular tissue, indicating that 2,6-DNT can be found in the lignin fraction. TNT is known to bind predominantly to the lignin fraction of wheat plants (15). The presence of the nitro peak at 1530 cm-1, CH3 peaks, and bands associated with the aromatic ring that appear in the 820–975 cm-1 suggest that 2,6-DNT was not fully transformed upon incorporation. Loss of the 1350 cm-1 band, however, shows that the compound is modified. No spectra are available for likely products such as aminonitrotoluene or hydroxyaminonitrotoluene. Limited metabolic capacity might explain why concentrations higher than 10 mg/L kill maize plants. We were able to demonstrate that SR-IMS can be used as a direct technique to determine fate and effects of organic compounds in plant tissue.
Acknowledgments
Funding for experimentation at the NSLS at Brookhaven National Laboratories was provided by the Department of Energy. Further support was provided by the U.S. Environmental Protection Agency under assistance agreement R-825550 through the Great Plains/Rocky Mountain Hazardous Substance Research Center and the Kansas Agricultural Experiment Station (contribution number 06-32-J), both in Manhattan, KS. The authors also would like to thank the staff at the NSLS at Brookhaven National Laboratories and the Kansas State University Veterinary Diagnostic Laboratory, Manhattan, KS, supervised by Cindy Chard-Bergstrom, for the use of their cryomicrotome.
K.M. Dokken and L.C. Davis are with the department of biochemistry at Kansas State University (Manhattan, KS). N.S. Marinkovic is with the Albert Einstein Center for Synchrotron Biosciences, Beamline U2B, National Synchrotron Light Source, at Brookhaven National Laboratory (Upton, NY). E-mail: dokken@ksu.edu
References
1. M.C. McCann, M. Bush, D. Milioni, P. Sado, N.J. Stacey, G. Catchpole, M. Defernez, N.C. Carpita, H. Höfte, P. Ulvskov, R.H. Wilson, and K. Roberts, Phytochem. 57, 811–821 (2001).
2. M.C. Jarvis and M.C. McCann, Plant Physiol. 38(1/2), 1–13 (2000).
3. J. Zeier and L. Schreiber, Planta 209, 537–542 (199).
4. J. Zeier and L. Schreiber, Plant Physiol. 113, 1223–1231 (1997).
5. T.K. Raab and J.P. Vogel, Infrared Phys. Technol. 45, 393–402 (2004).
6. T.K. Raab and M.C. Martin, Planta 213, 881–887 (2001).
7. D.L. Wetzel, A.J. Eilert, L.N. Pietrzak, S.S. Miller, and J.A. Sweat, Cell. Mol. Biol. 44, 145–167 (1998).
8. P. Yu, J.J. McKinnon, C.R. Christensen, and D.A. Christensen, J. Agric. Food Chem. 52, 1484–1494 (2004).
9. P. Yu, J.J. McKinnon, C.R. Christensen, D.A. Christensen, N.S. Marinkovic, and L.M. Miller, J. Agric. Food Chem. 51, 6062–6067 (2003).
10. S. Castro, L.C. Davis, and L.E. Erickson, Practic. Period. Hazard. Toxic Radioact. Waste Managem. 5, 141–152 (2001).
11. N.S. Marinkovic, R. Huang, P. Bromberg, M. Sullivan, J. Toomey, L.M. Miller, E. Sperber, S. Moshe, K.W. Jones, E. Chouparova, S. Lappi, S. Franzen, and M.R. Chance, J. Synchrot. Radiat. 9, 189–197 (2002).
12. N.S. Marinkovic, M.R. Chance, K.M. Dokken, L.C. Davis, D.H. Linkous, and J.M. Flinn, Am. Biotech. Lab. Jan., 12–13 (2005).
13. K.M. Dokken, L.C. Davis, L.E. Erickson, S. Castro-Diaz, and N.S. Marinkovic, Microchemical J. 81, 86–91 (2005).
14. B.A. Budevska, S.T. Sum, and T.J. Jones, Appl. Spectrosc. 57(2), 124–131 (2003).
15. C. Sens, P. Scheidemann, and D. Werner, Environ. Pollut. 104, 113–119 (1999).
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.
