Using XRF as an Alternative Technique to Plasma Spectrochemistry for the New USP and ICH Directives on Elemental Impurities in Pharmaceutical Materials
Spectroscopy
An evaluation of the ability of XRF spectrometry to perform elemental impurity analysis of 12 elements in various pharmaceutical materials
New guidelines governing the analysis of elemental impurities in pharmaceuticals are being implemented in the manufacturing of pharmaceutical products. X-ray fluorescence (XRF) spectrometry is a nondestructive analysis technique offering high sensitivity, precision, and accuracy without requiring chemical pretreatment. This installment of “Atomic Perspectives” evaluates the ability of XRF spectrometry to perform elemental impurity analysis of 12 elements in various pharmaceutical materials, such as cellulose, talc, and a mixture of cellulose, talc, and TiO2, by the calibration curve method using water solution standard samples, and verifying the qualification of United States Pharmacopeia (USP) Chapter <735>.
New guidelines governing the analysis of elemental impurities in pharmaceuticals are being implemented. Toxic heavy metals and residual metal catalysts may exist in the raw materials of active pharmaceutical ingredients (APIs) or be added during the manufacturing process, which may be a risk to human health.
In December 2014, The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) issued Q3D Step 4 Guidelines (1), which limits 24 elements, including Cd, Pb, As, Hg, V, Co, Ni, Ir, Pt, Rh, Ru, and Pd, in drug products and pharmaceutical ingredients. This guideline has now reached the implementation stage. In May 2015, the United States Pharmacopeia (USP) established X-ray fluorescence (XRF) methodology as General Chapter <735>, “X-ray Fluorescence Spectrometry.” XRF spectrometry is a nondestructive analysis technique offering high sensitivity, precision, and accuracy, typically without requiring lengthy chemical pretreatment (2).
This study assesses the capability of XRF to carry out the determination of these 12 elemental impurities in various pharmaceutical materials, including cellulose, talc, and a mixture of cellulose, talc, and TiO2, using aqueous-based calibration standards, and verifying the use of USP Chapter <735> as a screening tool for this analysis.
Permitted Daily Exposures and Concentration Limits
The ICH Q3D Step 4 Guidelines together with USP Chapter <232> “Elemental Impurities: Limits” (3), defines permitted daily exposure (PDE) values for oral, parenteral, and inhalational drug products. There are 24 elements included in the risk assessment and they are classified into four groups: Class 1 (Cd, Pb, As, and Hg), Class 2A (V, Co, and Ni), Class 2B (Tl, Au, Pd, Ir, Os, Rh, Ru, Se, Ag, and Pt), and Class 3 (Li, Sb, Ba, Mo, Cu, Sn, and Cr). Class 1 and 2A are very important elements and must be analyzed regardless of whether they are added intentionally or as result of the production process. Class 2B elements are not required to be checked for the risk assessment if these elements are not intentionally added; however, catalyst elements such as Ir, Pt, Rh, Ru, and Pd are commonly used in the process of producing APIs. It is necessary to convert the maximum daily intake to concentration limits because the PDE units are defined in micrograms per day. ICH Q3D Step 4 gives guidance in converting between PDEs and concentration limits as described below:
1. Option 1 is for common permitted concentration limits of elements across drug product components for drug products with daily intakes of no more than 10 g.
2. Option 2a is for common permitted concentration limits across drug product components for a drug product with a specified daily intake.
3. Option 2b is for permitted concentration limits of elements in individual components of a product with a specified daily intake.
4. Option 3 is for permitted concentration limits from finished product analysis. The most important thing is to change the concentration limits depending on the conversion method. Table I shows the oral concentration limits from PDE by options 1 and 2a for the assessment elements. The maximum daily intake assumes 1.0 g for option 2a.
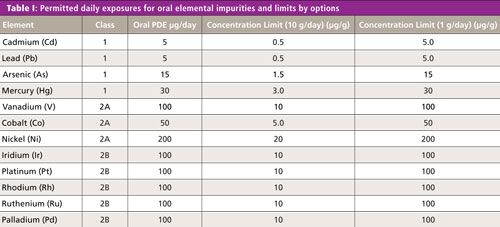
Table I shows oral drug PDE limits of Class 1, 2A, and 2B elements, together with the converted concentration limits based on 10 g and 1 g per day, daily dosages.
X-ray Fluorescence Spectrometry
The XRF technique, generally speaking, does not require chemical pretreatment, and since it is a nondestructive analysis it can identify and determine the concentrations of elements in solid, powdered, and liquid samples. XRF is capable of measuring a wide elemental range, at levels from below parts per million up to percent concentrations.
There are two types of XRF spectroscopic techniques: wavelength dispersive (WDXRF) and energy dispersive (EDXRF). EDXRF systems comprise an X-ray tube and a semiconductor detector and can simultaneously capture many types of fluorescence X-rays generated from the sample using a multichannel analyzer. EDXRF uses a lower power X-ray source, and will not significantly damage the sample. EDXRF systems are very convenient for users. In addition, EDXRF systems are smaller than WDXRF systems, they do not require any external utilities, such as chillers or gases, they typically use a 100-V power supply, and have no moving parts when measuring.
Quantitation is conducted using external calibration standards containing varying concentrations of elements. XRF calibrations have the advantage of being stable for long periods of time before requiring recalibration, in contrast to other elemental analysis techniques, such as inductively coupled plasma–optical emission spectrometry (ICP-OES) and ICP–mass spectrometry (MS) or atomic absorption spectroscopy (AAS). As the bulk of pharmaceutical materials are organic in nature, the EDXRF technique has the best potential for the nondestructive measurement of the aforementioned 12 elements in drug materials without the need for sample dissolution or chemical pretreatment.
Proposed Elemental Impurities Control Method for Drug Materials
USP Chapter <233> (4) specifies either the use of ICP-OES or ICP-MS for the determination of elemental impurities in pharmaceutical materials, or any other technique as long as it can meet the validation protocols. ICP techniques are typically more sensitive than XRF; however, they require chemical digestion techniques to dissolve the samples before measurements can be carried out. This process is very complicated and labor intensive and can be prone to human error and contamination of the sample. To reduce these time-consuming sample preparation procedures for ICP-OES and ICP-MS, the EDXRF technique can be used as a preliminary screening tool to determine if additional chemical analyses are required.
Furthermore, the XRF method may achieve similar performance for low-level chemical analysis if the measurement time is extended, because the detection limit improves with increasing sampling time. This makes EDXRF a very useful screening method to reduce analysis time and lower cost by minimizing the number of samples requiring precise analysis using ICP-OES or ICP-MS (5).
Sample Preparation
Solid, liquid, and powder samples can be analyzed by XRF with minimal sample preparation. The only preparation requirement is reducing the sample to a size that fits in the sample cell or chamber. Basically, the larger the sample volume, the smaller the sampling errors.
Almost all solid samples can be analyzed directly by simply placing them in the sample chamber, while liquid samples are poured in a sample cell, with a supporting film at the bottom. Typical films are made from polypropylene of a few micrometers thickness. The majority of drug materials are in organic matrices and have relatively low X-ray absorption, allowing measurement of the element impurities. Powder samples are placed directly into the sample cell using the tapping method to remove voids in the sample. Coarse powders must be ground to a fine particle size, and nonhomogenous samples should be ground by means of mortar and pestle or a grinding device, such as a mill.
Correction Method
XRF calibration is performed for each element by determining the relationship between measured intensities and concentration using a number of standard samples. In general, the measured intensity is corrected by some spectral deconvolution method because the fluorescence X-ray intensity is affected by sample matrices, sample volume, and sometimes interference from other peaks. These effects are known and well understood, and as a result, several methods have been developed to compensate for them, including the internal and overlap correction methods.
Performance Qualification of USP Chapter <735>
USP Chapter <735> was implemented in May 2015. The validation protocol includes operational qualification (OQ) and performance qualification (PQ) to verify that the system operates within target tolerances using appropriate samples with known spectral properties. OQ checks key operating parameters, such as peak position, detector resolution, and count rate, using specific calibration samples. The purpose of PQ is to determine that the instrument is capable of meeting the user’s requirements for all critical-to-quality measurements. These validation and verification protocols include linearity, accuracy and specificity, repeatability, intermediate precision, range, quantification limit, and robustness as described below.
Linearity
Analysts should demonstrate a linear relationship between the analyte concentration and corrected XRF response by preparing no fewer than five standards at concentrations that encompass the anticipated concentration of the test sample. The standard curve then should be evaluated using appropriate statistical methods such as least squares regression. The correlation coefficient (R), y-intercept, and slope of the regression line must be determined. Acceptance criteria: R is not less than 0.99.
Accuracy and Specificity
Analysts can determine accuracy by conducting recovery studies using the appropriate matrix spiked with known concentrations of elements. It also is an acceptable practice to compare assay results obtained using the XRF method under validation to those from an established analytical method. Acceptance criteria: 70–150% recovery.
Repeatability
Analysts should measure the concentrations of three replicates of three separate samples. Acceptance criteria: The relative standard deviation is not more than 20.0%.
Intermediate Precision
Analysts should establish the effect of random events on the method’s analytical precision. Typical variables include performing the analysis on different days, using different instrumentation, or having two or more analysts perform the method. As a minimum, any combination of at least two of these factors totaling six experiments will provide an estimation of intermediate precision. Acceptance criteria: The relative standard deviation (RSD) is not more than 25.0%.
Range
Range is the interval between the upper and lower concentration of an analyte in the sample, which has been demonstrated by meeting the accuracy requirement. 100%-centered acceptance criteria: The range is 80–120%. Noncentered acceptance criteria: 10% below the lower limit of specification to 10% above the upper limit of the specification.
Quantitation Limit
The limit of quantitation (LOQ) can be estimated by calculating the standard deviation of no less than six replicate measurements of a blank and multiplying by 10. Acceptance criteria: The analytical procedure should be capable of determining the analyte precisely and accurately at a level equivalent to 50% of the specification.
Robustness
The reliability of an analytical measurement should be demonstrated by deliberate changes to experimental parameters. Acceptance criteria: The measurement of a standard or sample response following a change in experimental parameters should differ from the same standard measured using established parameters by no more than ±20%.
Experimental
EDXRF spectroscopy measurements were carried out using an EDX-7000 benchtop spectrometer (Shimadzu Scientific Instruments). The spectrometer is equipped with an air-cooled X-ray tube (1000 μA, 50 kV, 50 W) and an electronically cooled silicon-drift detector. The calibration samples and unknown samples were measured using a counting time of 1800 s and an X-ray beam diameter of 10 mm.
The calibration samples were prepared by combining two aqueous stock solutions of Cd, Pb, As, Hg, V, Co, and Ni for Classes 1 and 2A, and Ir, Pt, Ru, Rh, and Pd for Class 2B. These standard solutions were prepared at more than five different concentrations including a blank by dilution with reagent water. The validation and verification samples were prepared from cellulose, talc, and a mixture of cellulose, talc, and titanium oxide powders spiked at three different concentrations with the aforementioned elements using standard solutions for AAS. The standard solutions were added to the above three types of blank powder samples and mixed in an agate mortar to prevent adhesion to the walls.
Next, 8.0 mL of each calibration standard solution together with 2.0-g powder samples were placed directly into a sample cell (32 mm diameter) with a 5-μm polypropylene support film. The powder samples were prepared using a tapping method to settle them in the cell in an effort to reduce voids in the sample packing. Then 1.0-g and 0.5-g powder samples were also placed directly into a sample cell using the tapping method with a 5-μm polypropylene support film for robustness testing. All concentrations of calibration samples were used to generate a calibration curve for each of the above elements. The range of calibration standards together with the validation and verification samples for each element are shown in Tables II and III.
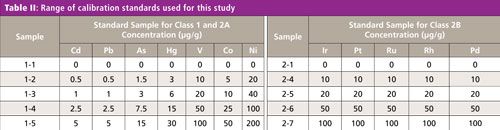
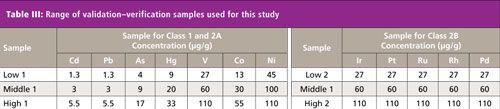
Continuous scattered X-rays at each specific energy range for the above elements were used as an internal standard to correct for sample matrix and volume effects. The quantitation of the impurity elements was calculated by the ratio of fluorescent X-rays for each element with continuous scattered X-rays.
Analytical Results
Linearity
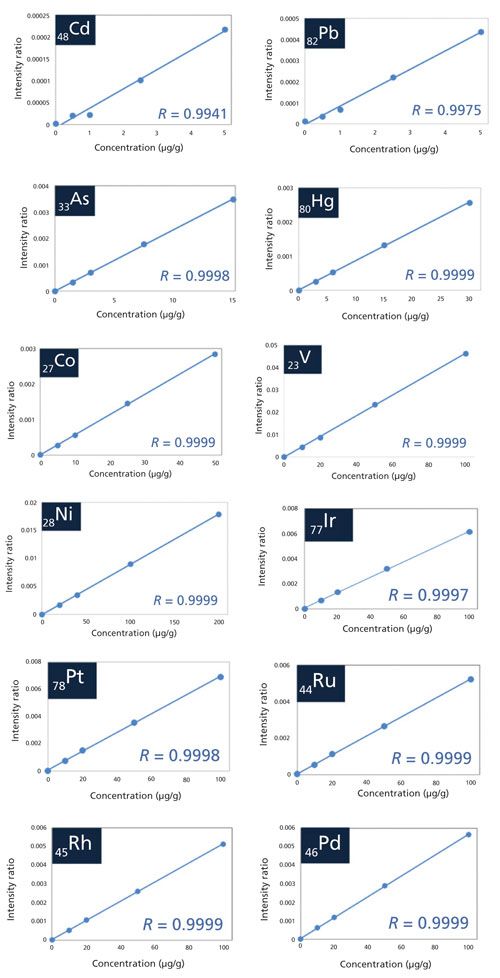
The correlation coefficient of all target elements for the calibration curve meets the acceptance criteria defined in the performance criteria section of USP Chapter <735> by using a minimum of five points and achieving a least squares correlation of at least 0.99. Overlap correction was used for As because the energies of AsKα (10.53 keV) and PbLα (10.55 keV) are very close together. The calibration curves for Cd, Pb, As, Hg, V, Co, Ni, Ir, Pt, Ru, Rh, and Pd are shown in Figure 1.
Figure 1: The calibration curves for Cd, Pb, As, Hg, V, Co, Ni, Ir, Pt, Ru, Rh, and Pd.
Please see the supplemental online information for Tables sI–sIX described below at: www.spectroscopyonline.com/supplementary-information-Atomic-Perspectives-July-2017.
Tables sI and sII show the accuracy and specificity and range results for Class 1, 2A, and 2B elemental impurities. For the range, the concentration of 100%-centered range (80–120%) defined the concentration limit at a maximum daily intake of 1.0 g from Chapter <232> oral drug PDE values; thus, the “High” sample is within the 100%-centered range. For this test, acceptance criteria for accuracy should be 70–150%.
The “Middle” samples are in the noncentered concentration range, and accuracy was interpreted such that it should be within the 63.0–165% range. However, the mixture sample could not validate or verify the V because of overlapping with TiKβ derived from the titanium oxide in the mixture sample.
For the talc sample, the elements at the low-energy region less than 10 eV, such as Hg, Ni, and V, have a low recovery rate because they are influenced by the matrix effect of other major components such as Si and Mg. However, the accuracy, specificity, and range of all target elements and matrices meet the acceptance criteria.
Repeatability
Tables sIII and sIV show the repeatability results. In this test, only the “Middle” samples were checked. The relative standard deviation was calculated from a total of nine data points for each material by measuring three replicates of three separate samples. All target elements and matrices meet the acceptance criteria of <20% RSD.
Intermediate Precision
Tables sV and sVI show the intermediate precision results. In this test, only the “Middle” samples were evaluated. The variable test evaluated two different days, three separate samples, two different EDXRF instruments and two different analysts. It is also valid to apply different combinations of the above events. For example, three analysts can be used if it is difficult to utilize more than two instruments. The relative standard deviation was calculated from a total of 12 data points for each event. All target elements meet the acceptance criteria of <25% RSD.
Quantitation Limits
The quantitation limit was calculated as the standard deviation of the intensity for 10 measurements of a blank cellulose sample. The quantitation limits for all target elements are shown in Table sVII for measurement times of 1800 s, with oral PDE values and control limits in the top four rows. In this investigation the control limit is defined as the PDE limit from ICH Q3D Step 4 Guidelines. The concentration limits shown are from conversion method option 2a, based on a dosage of 1 g per day. The two types of control values are based on USP Chapter <735> (50% of PDE) and the ICH Q3D Step 4 Guideline (30% of PDE). From these data, it can be concluded that the quantitation limit of the EDXRF technique can meet both control limits at 1.0 g maximum daily intake and can successfully perform screening measurements for elemental impurity control at these levels.
Robustness
USP Chapter <735> states that the reliability of an analytical measurement should be demonstrated by evaluating deliberate changes to experimental parameters. In this study, the experimental parameter was defined as sample volume. Tables sVIII and sIX exemplify the robustness results for three different sample volumes, showing the quantitation of 1.0 g and 0.5 g compared to 2.0 g. As demonstrated, all target elements meet the acceptance criteria of ±20%.
Conclusion
This study has shown that EDXRF meets USP Chapter <735> acceptance criteria for linearity, accuracy, specificity, range, repeatability, intermediate precision, quantitation limit, and robustness validation protocols. If 1 g per day is the maximum daily intake of a drug product, the technique can be applied to the screening and control of impurities for the most important risk assessment elements such as Cd, Pb, As, Hg, V, Co, and Ni, as well as for important catalyst elements including Ir, Pt, Ru, Rh, and Pd in pharmaceutical materials. The major benefit of the technique is that it reduces the need for complicated and time-consuming chemical digestion procedures and can be a very useful addition as an initial screening tool to reduce the number of samples that need to be analyzed by ICP-OES or ICP-MS.
References
- International Conference on Harmonization, ICH Q3D Step 4 Guidelines: Guideline for Elemental Impurities, (ICH, Geneva, Switzerland, 2014).
- General Chapter <735> “X-ray Fluorescence Spectrometry” in United States Pharmacopeia 37-National Formulary 32 (United States Pharmacopeial Convention, Rockville, Maryland, 2015).
- General Chapter <232> “Elemental Impurities - Limits” in United States Pharmacopeia 39-National Formulary 34 (United States Pharmacopeial Convention, Rockville, Maryland, 2016).
- General Chapter <233> “Elemental Impurities - Procedures” in United States Pharmacopeia 39-National Formulary 34 (United States Pharmacopeial Convention, Rockville, Maryland, 2016).
- “The Use of EDXRF for Pharmaceutical Material Elemental Impurity Analysis,” white paper, Shimadzu Scientific Instruments (2016).

Dan Davis is the Elemental Spectroscopy Product Manager for Shimadzu Scientific Instruments. He received a B.S. degree in Chemistry from Towson State University. He is responsible for managing the elemental spectroscopy products in North and Central America for Shimadzu including: ICP emission systems, atomic absorption systems, X-ray fluorescence and diffraction systems, and total organic carbon analyzers. He has more than 15 years of experience in the industry and contributes to ASTM and Standard Methods through review and submission of analytical methods.

Hiroaki Furukawa is the General Manager of the X-ray and Surface Analysis Business Unit for Shimadzu Corporation, Japan. He received a masters degree in Functional Materials from Niigata University. He is responsible for managing the electron probe microanalyzer systems, X-ray fluorescence and diffraction systems, and scanning probe microscope systems in Japan and overseas for Shimadzu. He has almost 20 years of experience in development and as an application engineer of X-ray products including total reflection X-ray fluorescence, X-ray reflectivity, and X-ray absorption fine structure.

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advanced Raman Spectroscopy Method Boosts Precision in Drug Component Detection
April 7th 2025Researchers in China have developed a rapid, non-destructive Raman spectroscopy method that accurately detects active components in complex drug formulations by combining advanced algorithms to eliminate noise and fluorescence interference.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.