The Write Stuff?
Guidelines for writing standard operating procedures in a laboratory and best practices for their use.

Written procedures used by trained staff are the basis for all quality systems, but there are many ways of writing a procedure. Have you got the "write" stuff?
A basic tenet of any quality system, be it ISO 9001, ISO 17025, good manufacturing practice (GMP) or good laboratory practice (GLP), is that we have qualified analysts and spectroscopists with the appropriate combination of education, experience, and training. Furthermore, they need to follow written procedures in their work, for example, analytical methods, preparation of mobile phases, weighing of reference and analytical standards, and sampling.
It is important to understand that there is no explicit statement in any quality system that mentions the quality of the output of the analytical laboratory. However, the principle used is that if qualified and trained staff follow written procedures then the analytical work and results should be better and more consistent than if there were nothing in place. For example, samples should be prepared the same way each time a specific analysis is performed: A mobile phase will be prepared consistently to eliminate run-to-run variation, samples presented to a sample probe will be consistently measured, and correction factors will be appropriately and consistently applied to calculated data. This is a good way to avoid out of specification (OOS) results.
The consistent element that runs through this whole stream is the written procedure. This document can be called a procedure, standard operating procedure (SOP), technical operating instruction, work instruction, or anything else that somebody thinks up. Regardless of the name, the written procedure is the key element that ensures that each analytical scientist is able to produce consistent work for the same process.
The problem is that there are many ways of writing a documented procedure — some are good, a few are very good, and some are bad. The purpose of this column is to look at ways procedures are written and how they could be improved upon the next time you are involved in reviewing them.
A Little Written Problem?
As an auditor I get to see some really amazing things in laboratories, but let's focus on the subject of this column. During one audit, I asked to see the procedure for a topic and was proudly presented with a pile of paper 117 pages long. You expect that there will be various document controls that take up some pages such as front pages with sign-off signatures, a table of contents, document history, but, even allowing for 3–5 pages of that, what remained was an awful lot of procedure. Looking through the document I noticed that there were no figures or tables, just text. Lots of text. In fact, lots and lots of text. More text on the subject than you could find in some textbooks. Certainly, more text than I really want to find in any procedure.
Christmas Arrives Early for the Auditor!
Auditing is not known for its excitement or entertainment value, either for the auditor or auditees. Despite this, there are times when the afternoon's entertainment is ensured (for the auditor) and this time it arrived in the form of a 117-page SOP. The only problem is that the auditor has to remember to keep a straight face.
Just think this through next time you consider writing a megaprocedure. When presented with a procedure of this length, consider the following questions:
- Has everybody read it? No!
- Of those who have read it, does anybody understand it? No!
- Is the procedure consistently followed? No!
I would go as far as saying that reading this SOP is highly unlikely to make anybody's bucket list.
So, the prologue of this entertainment can begin with the auditor asking to see the training records for the mega SOP. Presented with the training records you could expect to see some substantial training material for this procedure plus a demonstration of understanding or competence. Read and understand this procedure? Don't even think about this option, you'll need extensive training. Then you pick a lucky individual (better known to the auditor as the stooge) to bring in and begin "Act I."
The (un)lucky individual arrives. Fortunately, the auditor does not need to bring a selection of medieval torture instruments such as a rack or thumb screws because the laboratory had already provided one with the SOP. With a copy of the SOP on one side and the documented evidence of the output mandated by the procedure on the other, you ask the individual where in the procedure it states that a particular output must be produced. Just stay quiet and watch the entertainment unfold . . . "Act II" is simply "Act I" in reverse; here's what it says in the procedure, show me the evidence that this was followed. Everyone's a winner! If the auditor uses the power of silence effectively, the squirm factor index can rise substantially throughout this process.
Of course, the laboratory could have made your job even easier by updating the procedure the week before you arrived. In which case, the prologue becomes "Act I" as you'll be very interested to see how effective training has been undertaken in such a short period of time.
Lessons Learned
The key points to take away from this example is that to be effective procedures have to be read, understood, and followed. Therefore, less does equal more. Simple procedures that are understandable are the key. A procedure that is unreadable, complex, or not followed is worse than no procedure at all. The reason is that a laboratory understands the need for written procedures but has implemented them in a way that makes them impossible to follow, which defeats the purpose of the procedure. The problem is that you'll get kicked if you don't have a procedure, but you'll be kicked twice as hard if you have a procedure but don't follow it.
Writing a Procedure Is a Pain
The problem with writing or updating procedures is that it is a subject that is usually classified in the same category as watching paint dry. It is a pain every way you look at it, and it's perceived by many as a boring but necessary job to do. Typically, it is thought of as a job inflicted on the innocent analysts in the laboratory by those awful people who inhabit the evil planet of "Quality Assurance."
This is one end of the Gaussian distribution of analytical scientists working in the laboratory. Interestingly, these are the people who play the starring roles in quality audits and regulatory inspections by providing the ammunition to the auditors and inspectors to write their nonconformances or noncompliances, respectively. Perhaps it is worth reminding people with this attitude that the words above Traitor's Gate at the Tower of London say, "Abandon All Hope Ye Who Enter Here." Should that be written above the entrance to your laboratory?
Writing a Procedure Is a Business Benefit
At the other end of the Gaussian distribution are the analytical scientists who view written procedures as a benefit to a laboratory. Within a quality system, regardless of the standard or regulation you follow, there is a single set of requirements or rules that you have to follow, but there is room for interpretation. Therefore, all laboratories are the same, except for the differences. However, it is the differences that cause the problem. After all, how do you train new recruits, even from the same industry or quality system, in how you work? Perhaps by thought transference? Hence, the importance of effective, well-written, and presented SOPs. Put simply, the procedures describe how you work in your laboratory.
Essential Elements of a Written Procedure
What should be in an SOP or written procedure? Table I presents the main sections that should be in a procedure, which, inevitably, will vary from company to company but most have these sections with differing order. The purpose and scope sections should be fairly brief and outline why the procedure has been written and what it contains. Because we are discussing a description of an analytical process, key words and abbreviations should be defined, although a simpler alternative is to have a technical glossary for the whole laboratory that is simply cross-referenced within each procedure. A technical glossary is an easier and more elegant way to define technical terms and abbreviations and can even be subdivided into general analytical and technique specific terms and abbreviations in a single location that is easier to maintain.
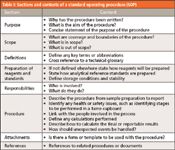
Table I: Sections and contents of a standard operating procedure (SOP)
Jumping to the bottom of the table is the references section, where there can be differences because some organizations will not put these into a procedure and others will. I am puzzled by the former attitude as most laboratories who do not cross reference other documents say it is to prevent an auditor or inspector from asking for other procedures. The main problem with this approach is that one of the first items asked for at the start of an audit is a list of SOPs that the laboratory has, which rather defeats the laboratory argument.
Because we are discussing procedures in an analytical laboratory, the preparation of reagents and analytical reference standards will be involved. If not defined elsewhere, the way of preparing these needs to be stated in the procedure, including a statement of the tolerances or ranges of critical standards. For example, it is unlikely that you will be able to weigh 20.0 mg of an analytical reference standard or a sample consistently, so the procedure should define what is acceptable — for example, 19.8–20.2 mg.
The main sections that I want to focus on in this column are the heart of any procedure: responsibilities and the procedure itself.
Know and Understand the Laboratory Process
Any procedure is written for a defined and repeated process not a one-off activity. Therefore, the key to writing an SOP is knowing and understanding the process that you are going to document. Sounds simple doesn't it? You would be amazed at the number of people who attempt to write a procedure without this knowledge. As such, you need an analytical scientist who is involved with the process. In the current technical jargon, this individual is called a subject matter expert (SME).
From the scope of the procedure, the boundaries of the procedure are defined along with the inputs (what do you need to start) and the outputs (what you end up with at the finish). Along the way there may be sample storage, preparation, analytical instrument setup, analysis and data recording, interpretation of the data, calculation of results, and reporting. What is covered by this specific procedure needs to be defined, and you need a person who understands what is going on.
Who Is Involved and What Do They Do?
The traditional way for writing a procedure is to consider the roles and responsibilities of the analytical staff that will be using the procedure in anger. We need to consider the following factors:
- Who is involved?
- What do they do at each stage of the process?
When there are hand-offs from one individual to another is where use problems could occur, and there is a need to define carefully which tasks must be completed by one individual and what must be passed on to another in terms of documentation and any records such as data files. One way to ensure that this transfer is complete is to use a checklist or a pro-forma document, although in regulated laboratories any pro-forma documents need to be formally issued and controlled. The other consideration is that most analytical work uses the four eyes principle: one person to perform the work and another one to review and approve it. It is important that one of the responsibilities of the performer is to ensure that the work package handed over for review is complete from the tester's perspective. So, glaring errors need to be trapped by the tester and not left for the reviewer to spot.
Ways to Write a Procedure
The typical way of writing a procedure is based on text. Here, the procedure needs to be clearly written because an SOP is an instructional document. Therefore, instructions must be written in the form of "do this" or "do that." The text needs to consist of short sentences that are written in simple, clear, and directive language. Long and complex sentences that could be misunderstood should not be used.
The effective use of white space is important for the impact on the reader and to clearly separate each individual instruction. Rather than save paper and run all the text into large paragraphs, the text needs to be spread out to separate individual instructions that make up the procedure. There will be empty lines between each instruction. Some companies number each instruction line and others don't; it's an organizational issue that probably goes back to when the quality system was first established.
Let us look at some instructions that could be in a written procedure for a laboratory. In Table II we have sample preparation instructions. They are written in a clear simple style: do this or do that. However, look at Table II to see the differences in the use of white space. Both columns have the same instructions for the sample preparation of samples for metals analysis by atomic absorption. The instructions in the left-hand column all run together and it is difficult to read or follow the procedure in the laboratory. This series of instructions is what you would see in a scientific publication of a method, but it is not appropriate for a laboratory procedure, in my view.

Table II: Using white space in a written procedure
In the right-hand column of Table II, the same instructions are broken up into a numerical list to illustrate the effective use of white space. The individual instructions can easily be followed, and that is how written procedures for either analytical methods or work in the laboratory should be set out.
Is everything good? No. Look at Table II, in the fourth bullet point in the right-hand column. It states either digest the same for 110 °C until it goes brown (all cooking is just good chemistry!) or if you are lazy and want to go home early then leave the samples at 40 °C overnight. Why are there two options? Has the method been validated to cope with either approach? In my opinion, the instructions should be a little clearer at this point.
Can we make the procedure any better? Yes, we can by the use of process-flow diagrams, an example of which is shown in Figure 1. The left-hand column shows a process-flow diagram of the procedure with each stage of the process shown as a box in the process flow. The same text from Table II has been inserted in the right-hand column without the numeric list and positioned next to the appropriate process flow activity to add detail to the process flow. So, this version of the procedure has a picture to give the overview and the text to provide the detail.

Figure 1: Procedure written using a process flow diagram with a process description.
We can develop this version of the procedure further by adding another column called responsibilities on the right to define who does each step and when the hand-over from one person to the next takes place can be described as well.
How Long Should a Procedure Be?
This is never an easy question to answer, but as short as possible is the best answer I can give. If necessary, have an overview procedure or guideline and then break a process down into a number of smaller ones that will be read and used rather than filed and forgotten. My ideal SOP length would be in the range of 3–5 pages including diagrams, the shorter the better providing that quality is not impacted.
How Do I Know You Followed the SOP?
In some quality systems there is the need to provide evidence that the procedure has been followed. In Chapter 4 of EU GMP (1) on documentation, there is the requirement for instructions (to perform a task, for example, SOP) and records that provide evidence of various actions taken to demonstrate compliance with instructions. In such cases, records are the result of procedures being followed. For an analytical procedure, this is relatively easy because it's the sample preparation and analysis records that can be on paper or in electronic format that demonstrate that the procedure was followed. For other procedures, the author needs to think about what evidence is generated by the procedure to provide evidence of compliance.
Training: Read and Understand or Assess Competence?
A classic method of training is the read-and-understand approach, where the procedure is distributed, either in paper or electronic form, to the analytical scientists who are going to use the procedure. Each person reads the procedure and then self-certifies that they have read and understood the procedure. Is this acceptable? It depends. If it is a new analytical procedure, probably not — you will want to have a better way to assess if an individual can perform the method and get acceptable results. If it is an update of an existing method with minor modifications, then read and understand is usually acceptable. However, the training world is moving from a read-and-understand methodology to demonstrating understanding or competence depending on the nature of the procedure involved.
Conclusions
In this installment, we looked at writing procedures that are instructions of how to perform an activity in a laboratory. Simple sentences with specific directions are needed with good use of white space to aid readability. Diagrams will help understanding and roles and responsibilities need to be defined within the procedure.
Reference
(1) Principle of Chapter 4 on Documentation, EU Good Manufacturing Practice, 2011.
R.D. McDowall is the principal of McDowall Consulting and the director of R.D. McDowall Limited, and the editor of the "Questions of Quality" column for LCGC Europe, Spectroscopy's sister magazine. Direct correspondence to: spectroscopyedit@advanstar.com.

R.D. McDowall
