A Dual Nanostructured Approach to SERS Substrates Amenable to Large-Scale Production
Surface enhanced Raman spectroscopy (SERS), although popular in research, has not yet been adopted in industrial applications. This is largely because of the challenge of manufacturing cost-effective, reproducible SERS substrates in volume that can be read by a compact reader for practical deployment in the field. Here we demonstrate and characterize a new dual-nanostructured SERS substrate approach that could be amenable to large-scale production. This dual-nanostructured surface combines long-range ordering of silver nanowires (AgNWs) with dense non-ordered decoration via silver nanoparticles (AgNPs) to improve on the SERS response characteristics of the individual structures, using fabrication methods that could be scaled for deposition via roll-to-roll Mayer rod coating. Characterization of the substrates’ SERS response using 638 nm Raman spectroscopy and ATP as the reporter molecule found the dual-nanostructured SERS substrate to offer both greater spatial uniformity and a consistently higher SERS enhancement factor (EF) than the nanowires alone.
Surface-enhanced Raman spectroscopy (SERS) is a technique whereby the Raman effect is enhanced by a factor of 106–108 or more through interaction of the analyte with noble metal nanostructures (1). This increases the limit of detection of Raman significantly for both quantitative and qualitative applications, and reduces performance demands on the associated instrumentation to allow greater deployment in the field. In SERS, the Raman signal enhancement originates from a combination of electromagnetic (EM) field enhancement because of the interaction of light with the nanostructures and the chemical enhancement (CE) that occurs when the analyte is chemisorbed on a SERS-active surface.
Common SERS-active structures include nanoparticles, nanowires, and nanostructured fabricated surfaces, with the greatest EM enhancement occurring in SERS hotspots, junctions between two nanostructures (1). Although both colloidal and solid SERS structures have been explored, solid substrates offer the greatest potential for commercial use, provided that they possess a sufficient number of SERS hotspots uniformly distributed within the spot size of the reader, in order to produce a reproducible, concentration-dependent signal while retaining high sensitivity. SERS substrates also allow convenient solution sampling, with the analyte becoming concentrated on the surface as the solvent evaporates to further increase interaction between the analyte and signal-enhancing substrate within the laser focus.
Both silver nanoparticles (AgNPs) and silver nanowires (AgNWs) are known to produce significant SERS signals, and can be synthesized relatively easily in the laboratory. However, the current fabrication methods utilize reaction conditions that pose challenges for large-scale production, from the need for meticulous controls over synthesis parameters to slow reaction times. This study demonstrates a SERS substrate where AgNWs and AgNPs are used as complementary nanostructures to improve on the SERS response characteristics of AgNWs alone, and where both structures are fabricated via easy, scalable synthetic methods.
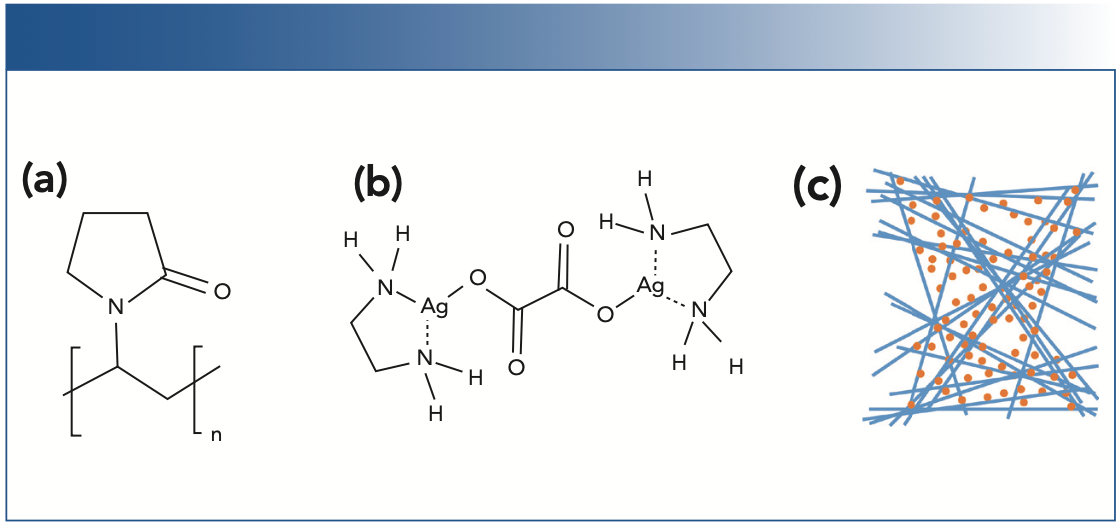
For the AgNWs, a simple one-pot synthesis with a fast reaction rate and low temperature is used, based on the popular “polyol” method. The polyol method utilizes hot ethylene glycol (EG) to reduce silver ions in the presence of polyvinylpyrrolidone (PVP, Figure 1a). The method is popular due to the use of environmentally-friendly reactants and relatively mild reaction conditions compared to other thermal solvolysis methods. Here, we employ FeCl3 as a source of Fe+3 to catalyze the reduction of silver and create shorter, thicker wires (2), as well as halide ions to promote wire formation.
For the AgNPs, a silver ion molecular complex is used as an alternative to traditional AgNP fabrication, in which a solid silver surface is rapidly produced when a diamminesilver(I) complex is exposed to an aldehyde functional group. The silver complex used here is μ-oxalatobis(ethylenediamine)silver(I), shown in Figure 1b. By utilizing a carboxylic acid as a ligand, gaseous CO2 is produced upon decomposition, and the decomposition reaction is slower. Because the reaction produces all gaseous organic species, a clean silver surface is produced absent of background interference from the capping agents often used to ensure colloidal stability (3), or additives required to avoid the coffee stain effect common in SERS substrates (uneven distribution of AgNPs resulting in concentrated rings on the surface) (4). Though the silver complex alone has previously failed to yield the morphology required for SERS response (5), the consistent formation of nanoparticles and known shelf stability (6) makes it a promising choice for incorporation with AgNWs into a dual-nanostructured SERS substrate.
FIGURE 1: (a) The structure of polyvinylpyrrolidone, used with hot ethylene glycol EG) to reduce Ag+ to form silver nanowires; (b) The structure of the silver complex μ-oxalatobis (ethylenediamine)silver(I) used to synthesize silver nanoparticles; (c), Schematic of the proposed dual-nanostructured substrate, composed of AgNWs decorated with AgNPs.
Additionally, by drawing on established industrial processing techniques like use of a whirlpool tank to isolate the AgNWs and roll-to-roll Mayer rod coating to deposit the SERS-active materials, a pathway is proposed for a SERS substrate which could be both manufacturable and cost-effective in volume.
Both AgNW substrates and AgNW substrates coated with AgNPs were fabricated using a variety of reaction parameters and deposition methods. These were evaluated to explore whether the easily produced AgNW coating could be utilized as a substrate to produce a uniform AgNP surface through the decomposition of an aqueous ink of the Ag complex on the AgNW substrate without the need of any additives, thus creating a dual-nanostructured surface. Results presented herein represent the optimized conditions relevant to demonstrating the dual-nanostructured surface; full details of the optimization process are presented elsewhere (7).
Materials and Methods
Silver Nanowire Synthesis
A synthetic method was developed to produce AgNWs based on the “polyol” method, incorporating a transition metal cation catalyst, and utilizing a selective precipitation purification method.
To synthesize the AgNWs, PVP (~150 mg) with a molecular weight of ~55,000 was dissolved in EG (20 mL ) with vigorous stirring, protected from light exposure. Silver nitrate was dissolved directly into the same reaction vessel, with a mole ratio of PVP monomer to silver of 2.0 to help direct one-dimensional growth of nanowires. An 11 mM stock solution of FeCl3 in EG (100 μL) was added with gentle swirling. The solution was heated to 100 °C in an oven for 45 min before increasing the temperature to 140 °C for 70 min, then cooled to room temperature.
The reaction products were dissolved in water (40 mL ) to create a homogenous dispersion. Acetone was then added slowly, dropwise with swirling, until a visible granularity was observed, and the mixture was allowed to settle for 15 min. The supernatant was removed, and water (~20 mL ) was added to resuspend the AgNWs. The selective precipitation was repeated 3–5 times, as necessary. Residual acetone and water were removed by centrifuging the solution at 3000 rpm for 3 min, removing the supernatant, redispersing in ethanol, and repeating the procedure four times before suspending the purified wires in a stock solution of ethanol (~5 mL ).
Qualitative analysis of the wire size distribution and purity was determined by bright- field optical microscopy using an Olympus BX60 microscope equipped with a DP73 color camera and polarization filters. SEM images were taken using a Hitachi SU8000 UHR FE-SEM. Composition and structure were characterized using UV-vis spectroscopy, thermal gravimetric analysis (TGA), and scanning electron microscopy (SEM), reported elsewhere (7).
Silver Complex Synthesis
The silver complex μ-oxalato-bis (ethylenediamine) silver(I) was synthesized as reported in literature (6). First, 0.5 M aqueous solutions of silver nitrate and oxalic acid were combined in an Ag+:oxalate volume ratio of 5:3. The silver oxalate precipitate was collected by filtration, washed with water, and dried at room temperature under vacuum. The silver oxalate was then dissolved in small increments, while stirring, in an aqueous solution of ethylene diamine (20% v/v), followed by precipitation with an excess of isopropanol, and subsequently filtered. The white precipitate was washed with cold isopropanol and vacuum dried at room temperature for 6 h prior to storage at -20 °C. The resulting silver complex was prepared as an ink for substrate fabrication by dissolving the silver complex in water (11 mg/mL). Care was taken to avoid light exposure during all synthetic steps and storage. The structure of the silver complex was confirmed via infrared (IR) and 13C nuclear magnetic resonance (NMR) spectroscopy, as well as TGA reported elsewhere (7).
Nanowire and Dual-Nanostructure Substrate Fabrication
Mayer rod coating was used to produce AgNW surfaces for direct use as a SERS substrate, herein referred to as a nanowire substrate (NWS), and for the fabrication of dual-nanostructured surfaces (DNS). To produce a NWS, the stock AgNW solution was pipetted (25 μL) in a line on glass slides and spread by hand using a #10 Mayer rod. The slides were dried at room temperature for ~30 min.
SERS quality DNS were produced by first preheating a NWS slide to 125 °C, then 25 μL of the Ag complex ink was drop-cast in the center of one half of the slide, and allowed to decompose for 5 min before cooling to room temperature, when it was then gently rinsed with ethanol and allowed to dry.
The uniformity of the fabricated surfaces was examined using an optical microscope. The morphology of the nanostructures on the surfaces were characterized using a Veeco Dimension 3100 AFM, operating in tapping mode.
SERS Characterization and Instrumentation
Aminothiolphenol (ATP) was used as the SERS reporter molecule for characterization of the substrates, drop-casted in small aliquots onto both the NWS and DNS sides of the SERS substrates, and air dried at room temperature for 2 min. Raman spectra were acquired with a fully integrated Wasatch Photonics 638 nm Raman spectroscopy system, utilizing a backscattering sampling geometry and spot size of ~60 μm, with detection via a fixed holographic transmission grating and a thermal electric-cooled (TEC) charge-coupled device (CCD) detector. SERS measurements were acquired using a laser power of 5 mW, taking 1 s per scan. Slide positioning was achieved using a Thorlabs MT3 XYZ stage controller equipped with micrometers to control translation (XY) and focus (Z) of the laser on the slide surfaces.
Results and Discussion
Nanowire Substrates (NWS)
Nanowire synthesis produced a narrow distribution of diameters of 80–120 nm, with lengths of 10–30 μm, as verified by optical microscopy, yielding an aspect ratio of ~200.
Notably, the reaction conditions employed required no control over stirring, injection rate, or other factors that would be difficult or expensive to incorporate into industrial scale reactions. The mechanical strength of the ~100 nm wires is enough to remain mostly straight with only a small amount of curvature, which bodes well for processability of the wires for substrate manufacture. The selective precipitation successfully isolates the desired AgNWs in a high purity and is readily adaptable to large scale execution in a whirlpool tank.
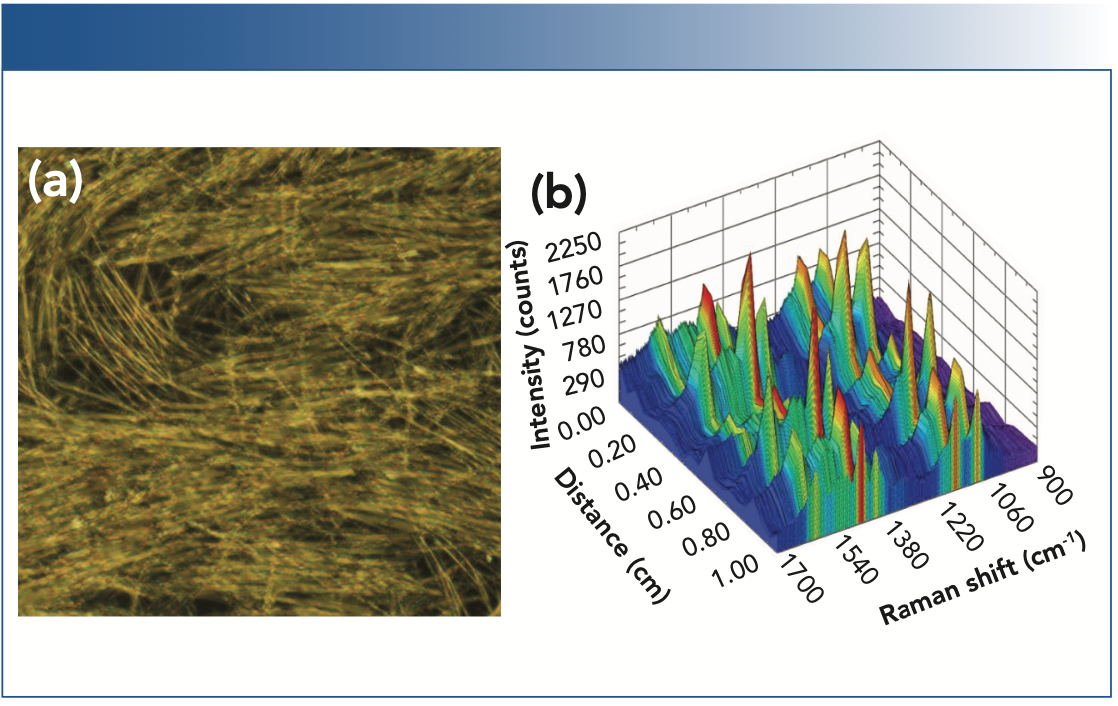
Deposition of AgNWs onto a slide via Mayer rod coating resulted in uniform coating and areas of linear structure with a high density of wire intersections to generate SERS-active hotspots, as imaged using microscopy (Figure 2a). To assess the uniformity of the SERS response in relation to spatial variations in sampling, SERS spectra of a 10 μL sample of 0.5 μM ATP solution were taken every 500 μm along a 1 cm linear path on the NWS, averaging four scans per location (Figure 2b). The SERS response of the NWS was found to be highly dependent on the position sampled, dropping to zero at some locations. The inconsistent spatial response of the NWS is a consequence of the AgNWs’ length being comparable to the spot size of the laser, which limits the number of SERS hotspots, as well as to gaps in deposition, that could be significantly improved in an industrial setting through the use of process automation.
FIGURE 2: (a) A microscope image of a typical nanowire substrate (NWS) produced by Mayer rod coating, showing both linear regions and gaps. (b) The SERS spectra acquired on the NWS over a spatial distance of 1 cm.
Dual-Nanostructured Substrates (DNS)
Because the AgNWs retain a surface layer of PVP as a byproduct of synthesis, it was hypothesized that the NWS could provide a guiding template for the deposition of a silver complex to populate the NWS with AgNPs in the interstitial space between and on the AgNWs, thus creating a dual-nanostructured surface with improved SERS characteristics, without the use of additives.
The NWS was then utilized as a substrate to produce a uniform AgNP surface via dropcasting and subsequent thermal decomposition of the aqueous ink of the Ag complex as the solvent evaporated. The outer edge of the deposited ink spot experienced a concentration of silver deposition due to the coffee stain effect, and thus all characterization was performed on the central area. While dropcasting was chosen to optimize SERS response for proof of concept, a pathway exists to adapt this step to Mayer rod coating for volume production (7).
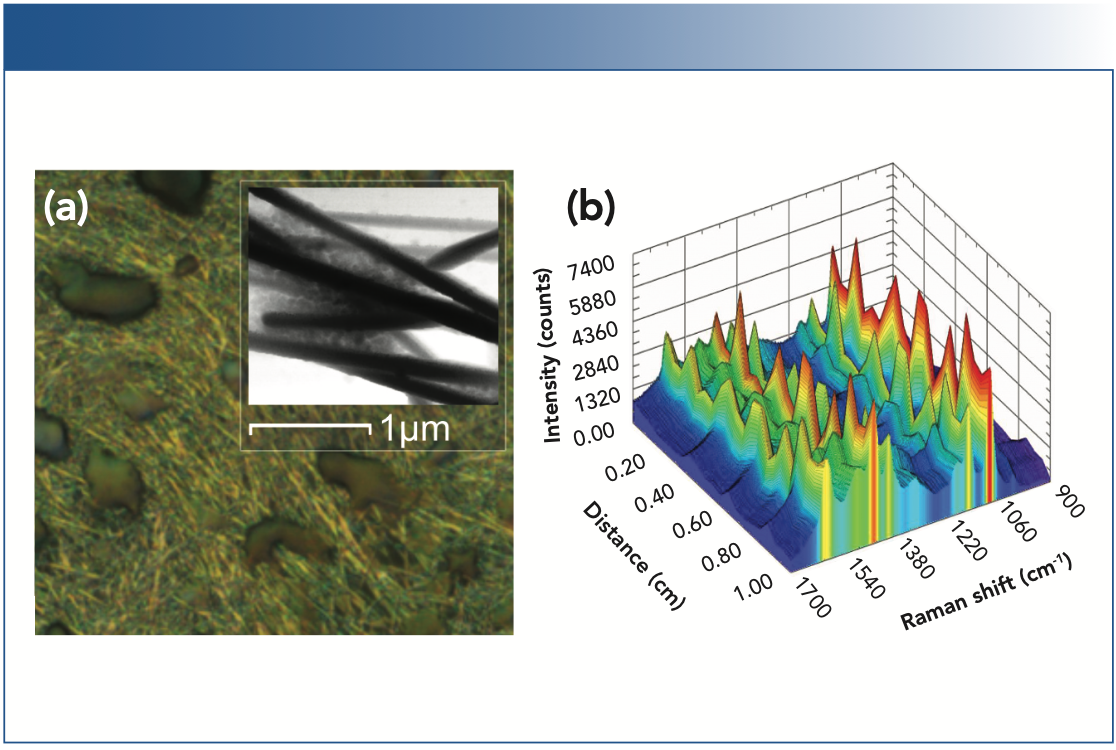
Optical microscopy showed craters interspersed with the AgNWs due to the elimination of the gaseous decomposition products (Figure 3a), while AFM revealed clusters of AgNPs at wire intersections, confirming the dual-nanostructured surface (Figure 3a, inset). The particles exhibit extremely small sizes and exist on a large range of Z heights. The same spatial uniformity assessment was applied to the DNS substrate, sampling SERS spectra of ATP every 500 μm along a 1 cm linear path for comparison to the NWS, averaging four scans per location (Figure 3b). The SERS response of the DNS retains some spatial fluctuations in the intensity of the acquired spectra, as expected due to variation in the underlying AgNW layer, but provides a minimum SERS response at every sampled location, as well as a consistently higher SERS response overall when compared to the NWS.
FIGURE 3: (a) A microscope image of the dual-nanostructured substrate (DNS) produced by drop casting the silver complex ink onto an NWS, with tapping mode AFM image (inset) showing nanoparticles filling the interstitial spaces between nanowires. (b) The SERS spectra acquired on the DNS over a spatial distance of 1 cm.
Augmenting the NWS with dense, nonordered AgNPs produced from a silver complex with good shelf stability (Figure 1b) resulted in an increase in the number of SERS hotspots. This approach improved the magnitude of the SERS response, while decreasing the spatial variability of the signal obtained from the AgNWs alone.
SERS Enhancement Factor
To quantify and compare enhancement factors (EF), the SERS spectra of a 5 μL sample of 423.5 μM ATP solution were taken at multiple locations of both a NWS and DNS fabricated on the same slide. Due to the spatial variability in the NWS, a total of 15 positions with local SERS signal max- ima were sampled, a process which took roughly 30 s per location. No optimization was needed for the DNS, and thus 10 random locations were sampled twice, at both t = 0 and t = 30 s, to capture the initial response, and to approximate the laser exposure time seen in the NWS sampling. Spectra are shown in Figure 4.
FIGURE 4: The SERS spectra of ATP for the nanowire substrate sampled at 15 locations and averaged (NWS), and for the dual-nanostructured substrate sampled at 10 locations, both initially (DNS-1) and after 30 sec (DNS-30).
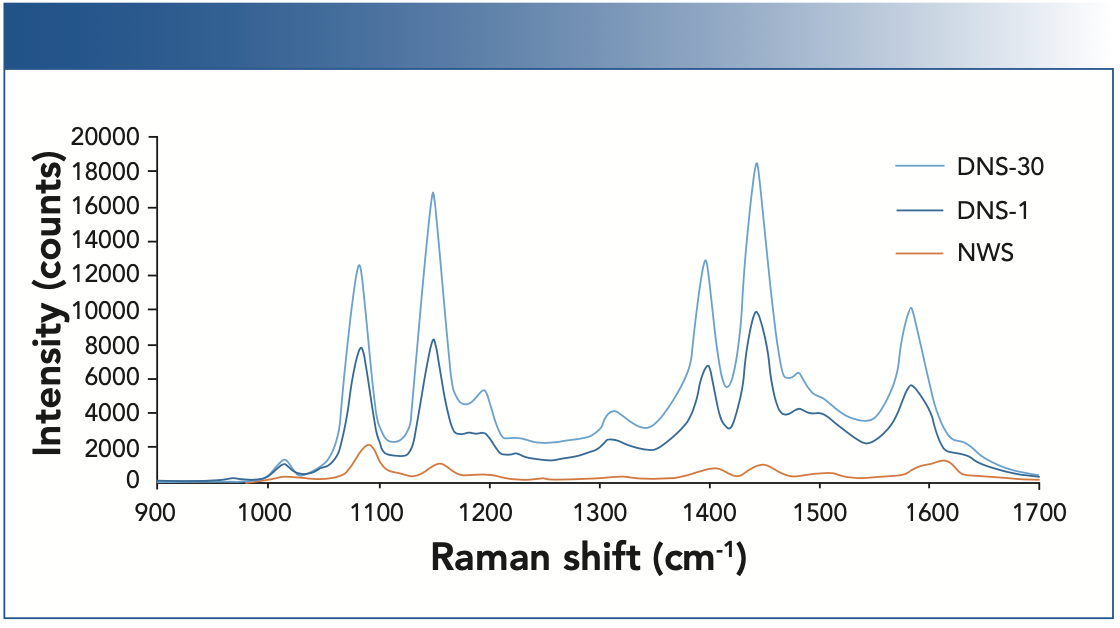
Peaks at 1084 cm-1 and 1600 cm-1 are due to vibrations of the aromatic ring of ATP, with contribution from the dimerization of ATP to 4,4’-dimercaptoazobenzene (DMAB) at 1084 cm-1 and 1584 cm-1 (8). The effects of ATP concentration and laser exposure on dimerization for these substrates were explored in more detail elsewhere (7). The calculated EFs for these peaks are shown in Table I. Peaks at 1150 cm-1, 1400 cm-1, and 1440 cm-1 were also observed, largely attributed to the selective enhancement of the b2 vibrational modes of ATP during SERS analysis (9,10).
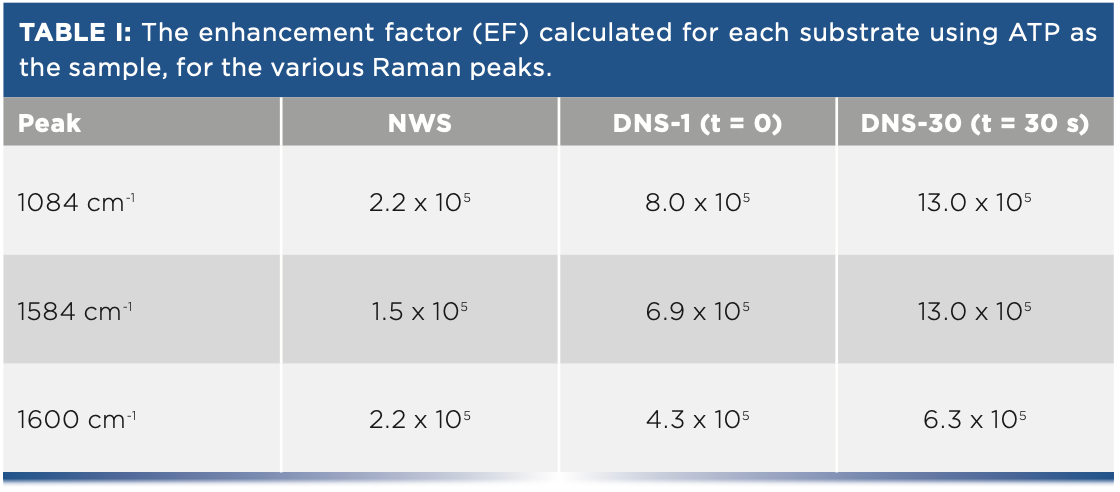
Although the contribution from DMAB to the spectrum makes it difficult to reliably quantify the increase in EF for the DNS vs. the NWS (7), all substrates demonstrate an EF of 105 or greater, with the dual-nanostructured substrate showing a clear improvement in EF over the nanowire substrate.
Conclusion
A nanowire substrate utilizing AgNWs and a dual-nanostructured SERS surface that also incorporated AgNPs were fabricated using synthesis methods and production processes amenable to cost-effective volume production. The AgNWs synthesized were of high purity and narrow size distribution, ~10 μm in length and ~100 nm in diameter, and were deposited using Mayer rod coating to form a SERS-active substrate. Although high spatial variation in the number of SERS hotspots relative to the spot size of the laser limits its applicability as a quantitative SERS device, the AgNWs were shown to be an effective substrate to facilitate subsequent dropcasting with an aqueous solution of a silver complex to populate the interstitial spaces between the AgNWs and improve the SERS response using an additive-free process.
The resulting DNS exhibited both improved spatial uniformity in the SERS response compared to the NWS alone, as well as a significant increase in intensity. Through further process development and adaptation of AgNP deposition to a Mayer rod coating method, the dual-nanostructured SERS approach offers a pathway to industrial fabrication of a low-cost, SERS-active substrate.
References
(1) C.L. Haynes, A.D. McFarland, and R.P. Van Duyne, Anal. Chem. 77(17), 338A–346A (2005).
(2) K. Zhan, R. Su, S. Bai, Z. Yu, N. Cheng, C. Wang, S. Xu, W. Liu, S. Guo, and X.-Z. Zhao, Nanoscale 8(42), 18121–18133 (2016).
(3) W.E. Smith, Chem. Soc. Rev. 37(5), 955–964 (2008).
(4) A.T. Fafarman, S.-H. Hong, S.J. Oh, H. Caglayan, X. Ye, B.T. Diroll, N. Engheta, C.B. Murray, and C.R. Kagan, ACS Nano. 8(3), 2746–2754 (2014).
(5) X. Liu, C. Zong, K. Ai, W. He, and L. Lu, ACS Appl. Mater. Interfaces 4(12), 6599– 6608 (2012).
(6) K.R. Zope, D. Cormier, and S.A. Williams, ACS Appl. Mater. Interfaces 10(4), 3830–3837 (2018).
(7) K.B. Castro, M.Sc. dissertation, Old Dominion University, Norfolk, Virginia (2019).
(8) Y.-F. Huang, H.-P. Zhu, G.-K. Liu, D.-Y. Wu, B. Ren, and Z.-Q. Tian, J. Am. Chem. Soc. 132(27), 9244–9246 (2010).
(9) K. Uetsuki, P. Verma, T.-a. Yano, Y. Saito, T. Ichimura, and S. Kawata, J. Phys. Chem. C 114(16), 7515–7520 (2010).
(10) Y.-C. Chen, J.-H. Hsu, and Y.-K. Hsu, Microchim. Acta 185(2), 1–8 (2018).
Kory Brian Castro and John Cooper are with the Department of Chemistry at Old Dominion University, in Norfolk, Virginia. Cicely Rathmell is Vice President of Marketing at Wasatch Photonics, in Morrisville, North Carolina. Direct correspondence to: marketing@wasatchphotonics.com
AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.
