A Furfural Derivative–Indolium Conjugated Colorimetric and Fluorescent Probe for Cyanide Detection
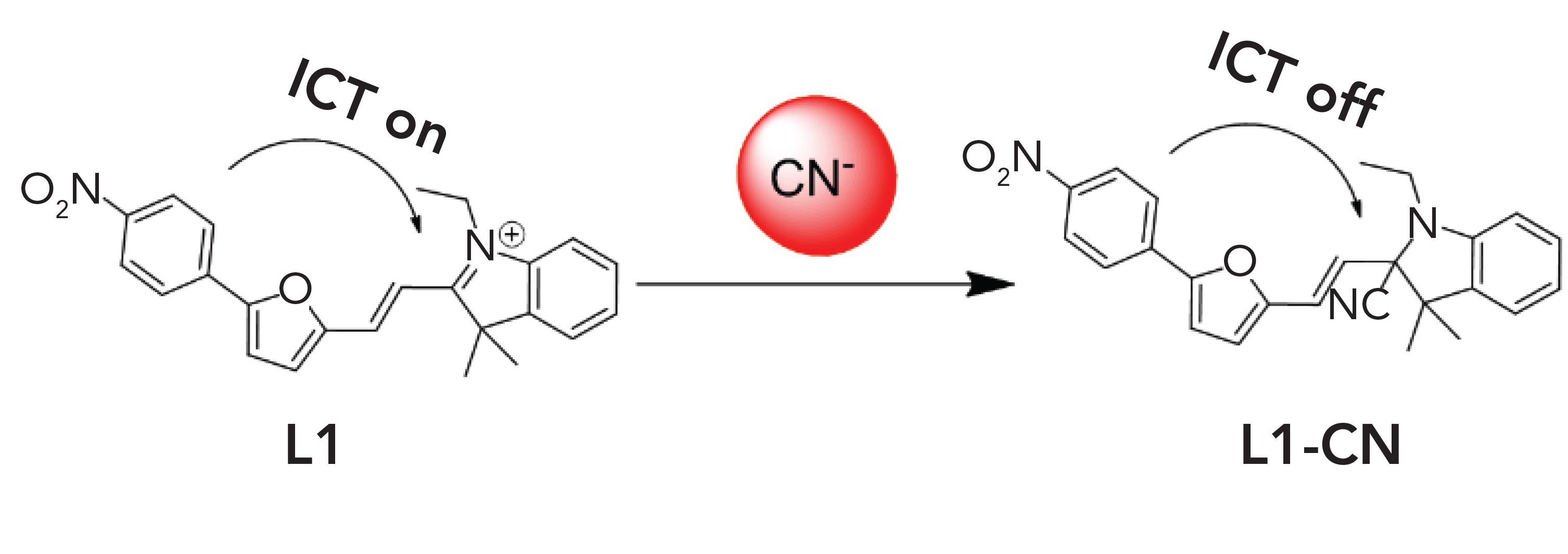
A novel furfural derivative–indolium conjugated colorimetric and fluorescent probe, called L1, has been synthesized via the aldol reaction of 5-(p-nitrophenyl) furfural and 1-ethyl-2,3,3-trimethyl-3H-indol-1-ium iodide, and its response to cyanide in a DMSO/H2O (1:1, v/v) solution was measured using colorimetric and fluorescence analysis. This L1 probe showed a significant solution color and fluorescence change in response to cyanide, even in the presence of other anions, such as F-, Cl-, Br-, I-, Ac-, SO42-, H2PO4-, ClO4-, S2- , and SCN-. In UV-vis and fluorescence spectrometry analysis, a large blue shift (83 nm), color bleaching, and fluorescence quenching were observed upon addition of cyanide anion (CN-). A significant color change was detected by visual observation. Additionally, the limit of detection of the L1 probe toward CN- was 3.65 × 10-7 mol/L. This result was attributable to an intramolecular charge transfer (ICT) in the probe. The ICT progress was then terminated, along with the color change and fluorescence quenching, as the indol C=N underwent a nucleophilic attack of CN-. The CN- detection mechanism of the probe was confirmed by 1H NMR, mass spectrometry (MS), and density functional theory (DFT) measurements.
In recent years, more attention has been paid to the design and preparation of small-molecule fluorescent probes to detect and recognize anions in the area of optical materials (1–5). Among many anions, cyanide anion (CN-) is important as a result of its special characteristics (6,7). CN- is widely used in organic synthesis, metallurgical engineering, electroplating technology, and other fields (8–10). The increased used of CN- in industry has also led to increased cyanide contamination in the environment (11,12). Cyanide is one of the most highly toxic compounds and it can cause harm to living organisms, decreasing oxidative metabolism utilization of oxygen; Fe3+ in heme has a preference for CN-, leading to the phenomenon of oxygen deficiency (13–15). As a result, it is essential to develop new detection methods to trace CN- in the environment.
Titration, electrochemistry, and spectroscopy have been widely used to detect CN- (12,16–18). Spectroscopic methods in particular have attracted increasing attention for this purpose, because of their higher selectivity, higher efficiency, easier operation, and lower cost compared to the other methods (19–21). It has been reported that a variety of colorimetric and fluorescent probes exhibit alteration of absorbance or fluorescence upon interaction with CN-, because of reactions that lead to cyanide addition on many functional groups, such as thiazole (15,22), aldehyde (23), Schiff base (18, 24–28), dicyano-vinyl (19, 29–32), and imidazole.
In this work, we synthesized a furfural derivative–indolium conjugate, called L1, for use as a cyanide probe. The conjugate is based on 5-(p-nitrophenyl) furfural and 1-ethyl-2,3,3-trimethyl-3H-indol-1-ium iodide in a DMSO/H2O (1:1, v/v, Tris-HCl buffer, pH 7.2) solution (scheme 1). The limit of detection (LOD) of the L1 probe for CN- was 3.65 × 10-7 mol/L, far surpassing the World health Organization (WHO) standard (1.9 × 10-6 mol/L) (33). The probe's response to CN-, based on a deprotonation process that the indolium C=N group of the L1 probe underwent during the nucleophilic attack of CN-, retards conjugation between the furfural derivative and the indolium compound, resulting in color and spectroscopic changes. The solution color faded gradually, and the fluorescence quenched slowly, with the dropwise addition of cyanide and the probe readily responded to the cyanide concentration even in the presence of other anions, such as F-, Cl-, Br-, I-, Ac-, SO42-, H2PO4-, ClO4-, S2-, and SCN-. The detection mechanism of the probe L1 for CN- was confirmed by 1H NMR, mass spectrometry (MS), and density functional theory (DFT) measurements.
SCHEME 1: Detection of CN- by the L1 probe.
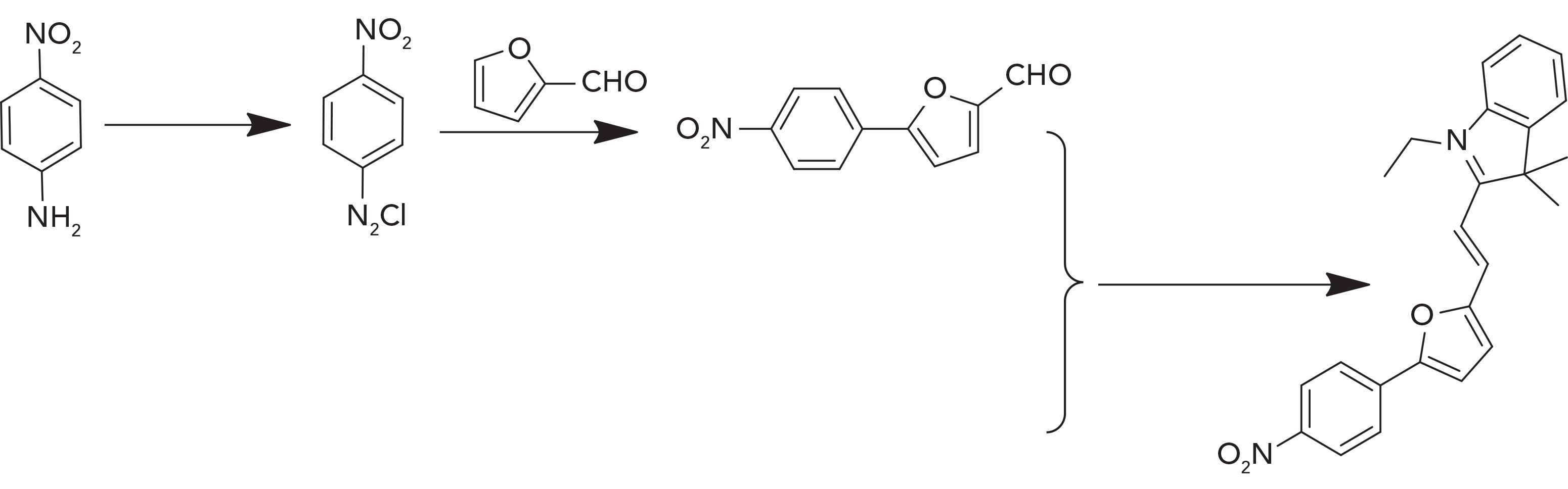
SCHEME 2: Steps in the synthesis of the L1 probe: (1) NaNO2, HCl, 0–5 °C. (2) CuCl2, 70%. (3) acetonitrile, refluxed, 70%. (4) piperidine, EtOH, refluxed, 72%.
Materials and Methods
Chemicals and Apparatus
All reagents and solvents were analytical grade, and used as obtained from commercial sources without further purification. Ultrapure water was used throughout the experiments. Tetrabutylammonium salt of anions (CN-, F-, Cl-, Br-, I-, Ac-, SO42-, H2PO4-, and ClO4-) were purchased from Sigma-Aldrich. Sodium salts (S2- and SCN-) were obtained from Energy Chemical. 1H NMR and 13C NMR spectra were obtained using a 500 MHz instrument and TMS was used as an internal standard. A Shimadzu UV-2550 spectrometer was used to produce UV-vis spectra. Fluorescence spectra were obtained using a Shimadzu RF-5301 fluorescence spectrophotometer.
Synthesis of the L1 Probe
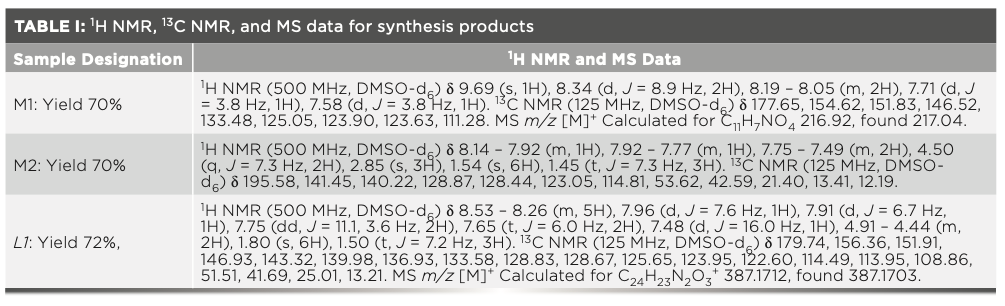
A schematic illustration of the steps in the synthesis of the L1 probe is shown in Scheme 2. First, 5-(p-nitrophenyl) furfural (M1) and 1-ethyl-2,3,3-trimethyl-3H- indol-1-ium iodide (M2) were prepared as described in previous work (34,35). Then, 5-(p-nitrophenyl) furfural (2mM) and 1-ethyl-2,3,3-trimethyl-3H-indol-1-ium iodide (2 mM) were mixed in absolute ethanol (20 mL). Piperidine was added to the solution as a catalyst. The mixture was stirred and refluxed at 80 °C for 12 h. After the above reaction, the solution was cooled to ambient temperature immediately; after removing the solvent and following purification, a dark red powder, which is the L1 probe, was obtained. The 1H NMR spectra data for the various synthesized materials is given in Table I.
Theoretical Approach to the structure of the L1 Probe and L1-CN
The Gaussian 09 program was performed in order to compute a theoretical structure for the probe. The optimization of the molecular configurations of L1 and the additive product L1-CN in the ground and excited states were carried out using DFT with the b3lyp methodology involving 6-31G (d) basis sets.
Results and Discussion
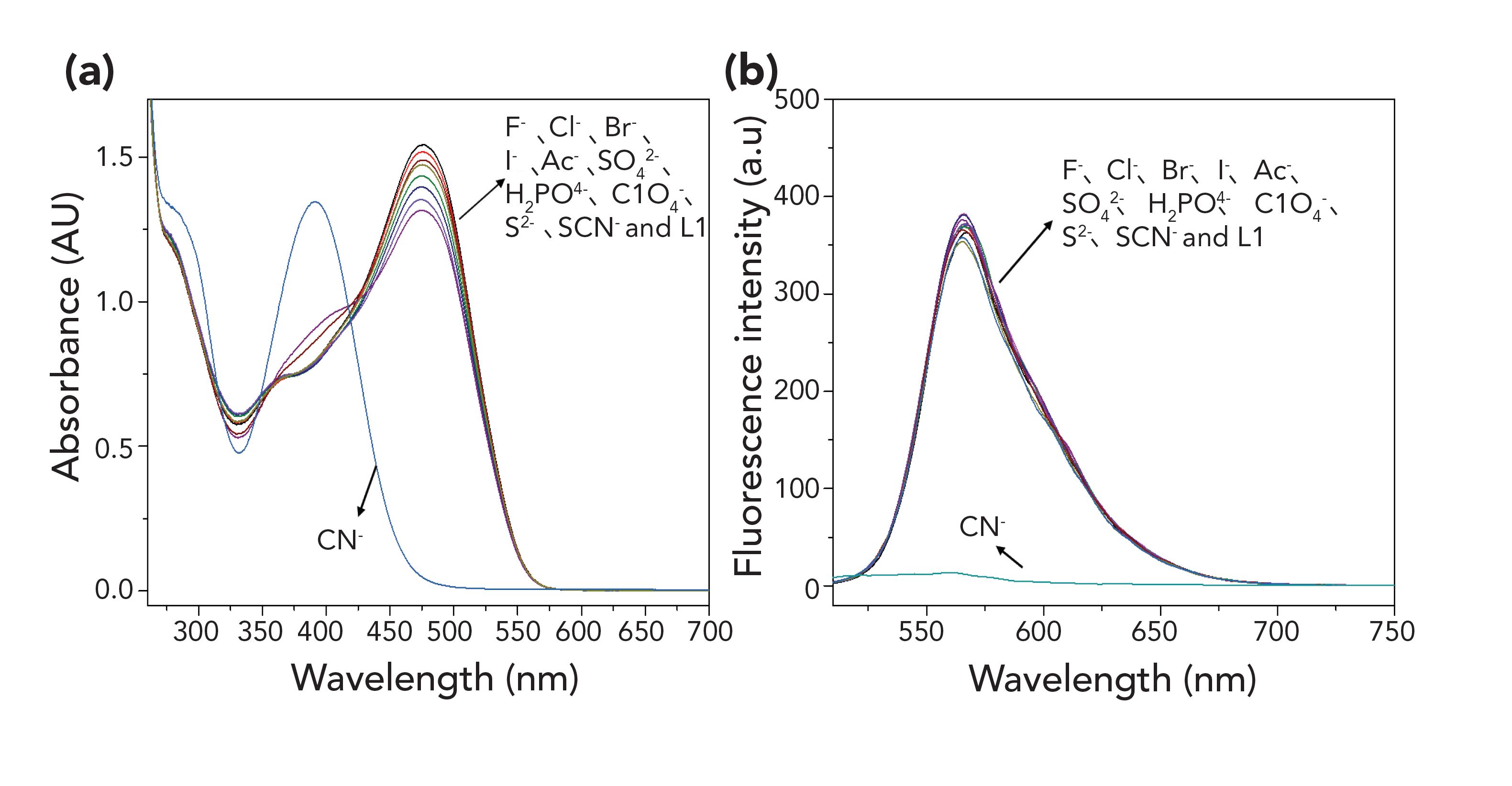
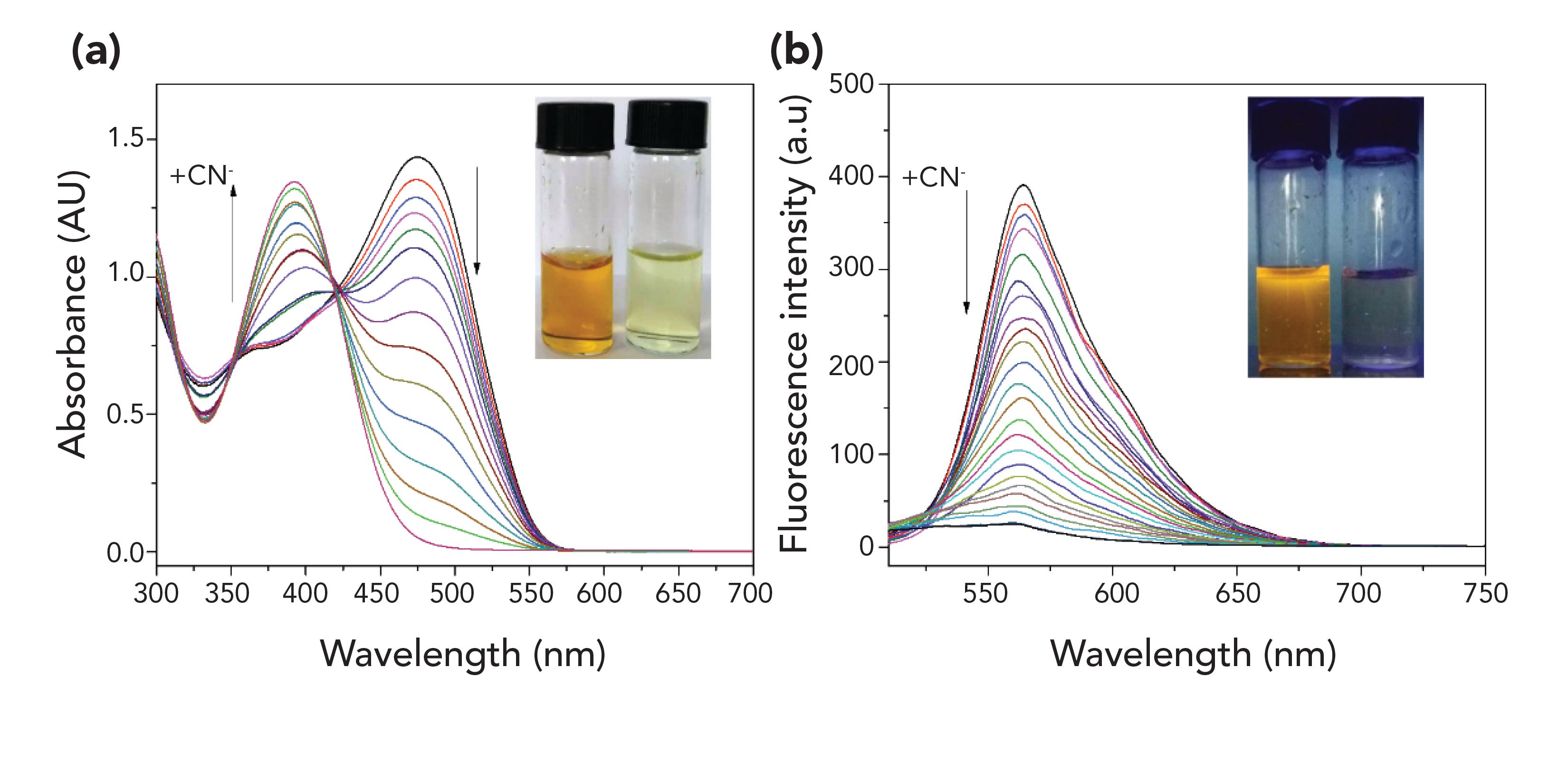
The L1 probe was prepared to have a strong electron-withdrawing benzpyrole derivative and an electron donating fur-aldehyde derivative, which are connected through an ethylene bridge, and then form a typical "D-π-A" conjugation system with intramolecular charge transfer (ICT) properties. Evaluation of the structure of the L1 probe was achieved by analysis of 1H NMR, 13C NMR, and MS spectra. Detailed characterization of the intermediates (M1 and M2) and product (L1) are provided in the supporting information from Table I. The selective behavior of the L1 probe toward anions was tested with UV-vis absorption spectroscopy and fluorescence spectroscopy in a DMSO:H2O (1:1, v/v, 0.01 M Tris-HCl buffer, pH 7.2) solution. The UV-vis absorption spectral response of the L1 probe was tested with aqueous solutions of the salts of all common anions analytes, including CN-, F-, Cl-, Br-, I-, Ac-,SO42-,H2PO4-, ClO4-, S2-, and SCN-. The L1 probe displays a strong absorption peak, with a maximum at 475 nm (Figure 1a). The addition of 50 equivalents of other anions did not cause any significant absorption band changes at 392 nm with a gradually increasing concentration of CN-. In the same manner, the L1 probe displays a strongly fluorescence peak at 565 nm (Figure 1b). Upon increasing the concentration of CN-, the fluorescence peak disappears, and other anions did not cause significant fluorescence changes.
FIGURE 1: (a) UV–vis absorption spectra of the L1 probe (2.0 × 10−5 M) with analytes DMSO/H2O (1:1, v/v) in the presence of CN , F , Cl , Br , - - 2- - - 2- - I,Ac,SO4 ,H2PO4 ,ClO4 ,S andSCN.(b)FluorescencespectraoftheL1 probe (2.0 ×10−5 M) with various analytes in DMSO/H2O (1:1, v/v).
To obtain more information about the response of L1 to CN-, we carried out UV-vis and fluorescence titration experiments. As the concentration of CN- was gradually increased, the UV-vis absorption at 392 nm increased, while the absorption gradually decreased at 475 nm (Figure 2a). It is noteworthy that the blue shift of the absorption wavelength, consistent with an evident color change from yellow to lime, demonstrates that L1 can achieve the goal of detecting CN- in a manner that is visible to the naked eye. The absorption intensities at 475 nm decreased linearly with an increasing CN- concentration from 0.0 to 25 μM (R = 0.995), and the achieved detection limit was 3.65× 10-7 results proved that the L1 probe could be used as an effective sensor for monitoring trace amounts of CN- based on colorimetric and fluorescence analysis.
FIGURE 2: (a) UV–vis spectra of L1 (2.0 × 10−5 M) in DMSO/H2O (1:1, v/v) [0.01 M Tris-HCl buffer, pH 7.2] upon adding an increasing concentration of CN−. Inset: color change of solution. (b) Fluorescence spectra of L1 upon adding an increasing concentration of CN−. Inset: color change of the solution.
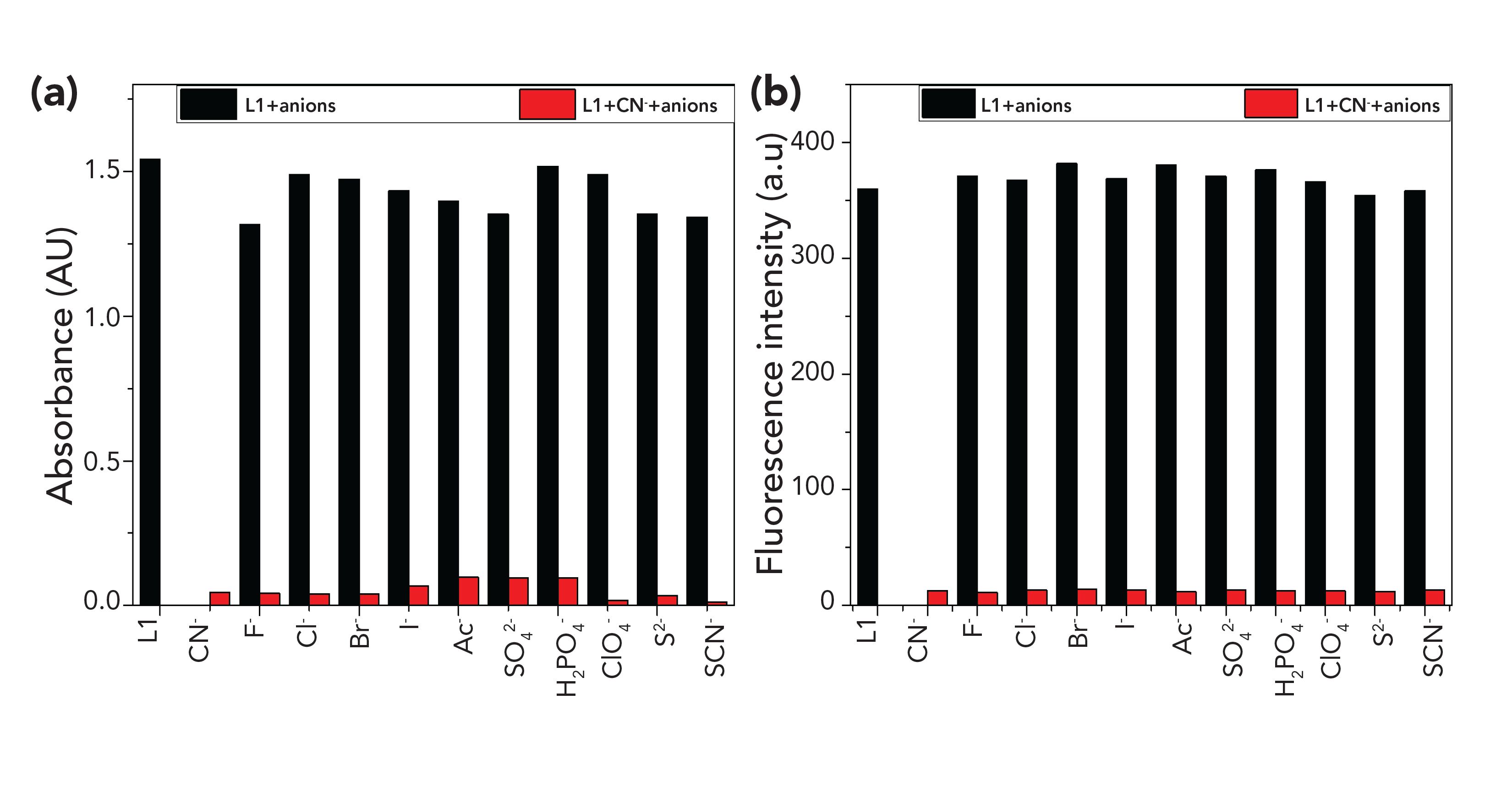
UV-vis and fluorescence spectroscopy were used to evaluate the selectivity of L1 to CN- over other anions. The addition of 50 equivalents of CN- and other anions, including F-, Cl-, Br-, I-, Ac-, SO42-, H2PO4-, ClO4-, S2-, and SCN-, to L1 did not lead to observable changes in absorption at 475 nm or the intensity of emission at 565 nm (Figure 3); the color change and fluorescence quenching of L1 toward CN- remained apparent, and little interference from other anions was noted.
FIGURE 3: (a) UV–vis spectra of L1 at 475 nm with addition of CN- in the presence of 50 equivalents of other anions (0 L1; 1 CN-; 2 F-; 3 Cl-; 4 Br-; 5 I-; 6 Ac-; 7SO 2-;8H PO -;9ClO -;10S2-;11SCN-)inDMSO:H O(1:1,v/v). (b) Fluorescence of L1 at 565 nm with addition of CN- in the presence of 50 equivalents of other anions in DMSO:H2O (1:1, v/v).
To examine the possible mechanism of the L1 probe's ability to detect CN-, 1H NMR titration, MS, and DFT methods were carried out. For the 1H NMR titration experiment, L1 and TBACN were dissolved in DMSO-d6. Prior to the addition of CN-, the 1H NMR chemical shift of the vinylic proton Ha and Hb on L1 was at 7.97 ppm and 7.91 ppm. The–N–CH2- of the Hc protons was at 4.76 ppm. After CN- was added into the L1, all 1H NMR signals shifted upfield. The peaks belonging to the vinylic protons Ha and Hb exhibited an upfield shift from 7.97 and 7.91 ppm to 6.75 and 6.48 ppm upon adding 1 equivalent of CN-. Additionally, the signals in accordance with –N–CH2– (Hc protons) presented an upfield shift from 4.76 to 3.17 ppm (Figure 4c). Further, MS analysis was carried out. The L1 peak was at 387.32 m/z (Figure 4b). The peak of the additive product L1-CN was at 414.36 m/z (Figure 4a). The additive product L1-CN was characterized upon adding 1 equivalent of CN- to L1. 1H NMR and MS data indicated that L1 underwent the cyanide nucleophilic addition reaction that cleanly converted the -C=N group.
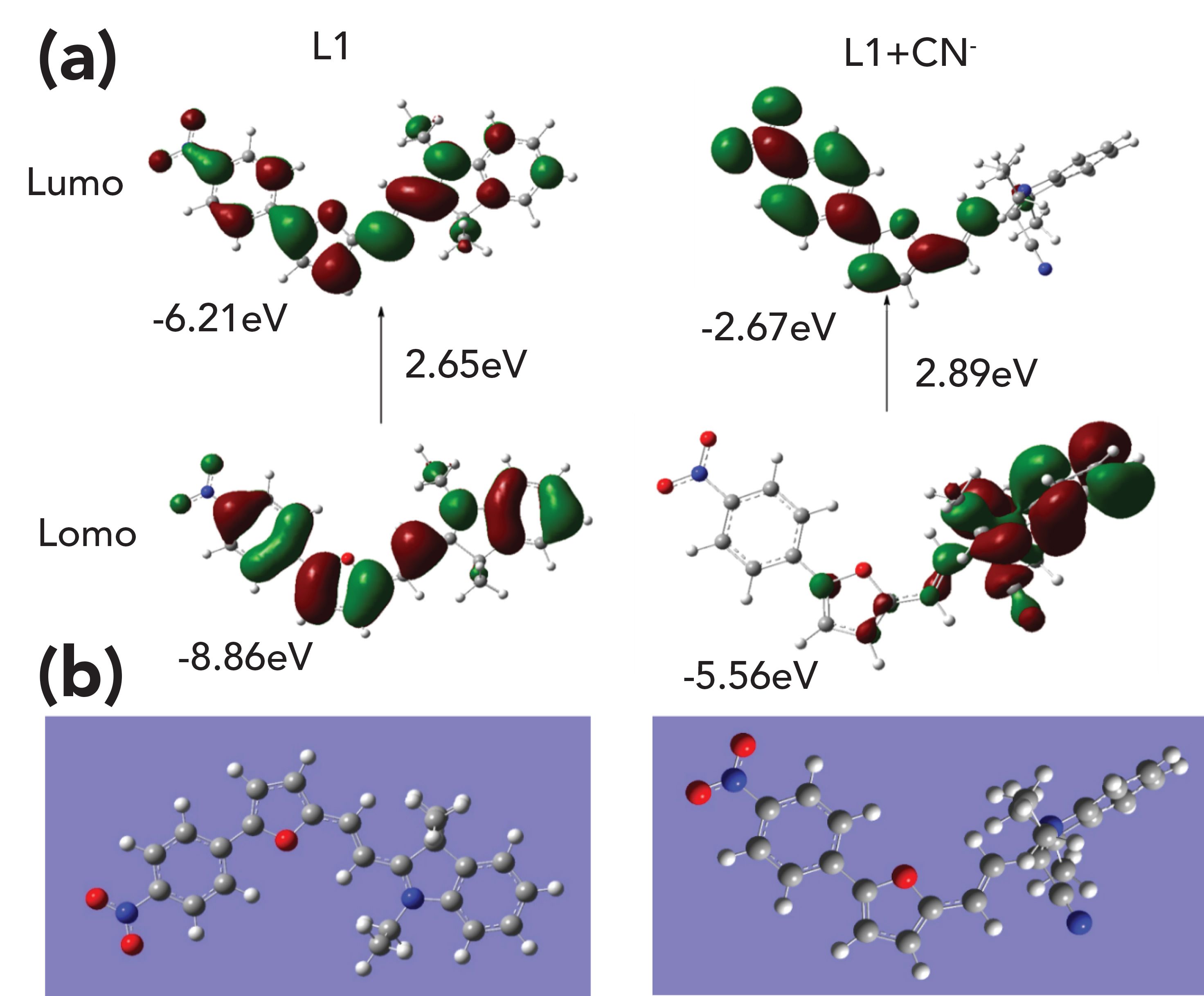
To validate the suggested mechanism of L1 detection of CN-, DFT calculations were made at the b3lyp /6-31G(d) level of theory. In Figure 5, the optimized ground-state (S0) structures, the highest occupied molecular orbitals (HOMO), and lowest unoccupied molecular orbitals (LUMO) of L1 and L1-CN are shown. Important differences between L1 and L1-CN can be seen. Both the HOMO and LUMO of L1 disperse over the whole molecule, demonstrating good delocalization of the electron cloud density and the active ICT processes. Upon the nucleophilic addition of CN-, the conjugation is interrupted, the ICT process is blocked, and the HOMO and LUMO of the L1-CN additive product are focused on the furfural derivative and indolium. Also, the HOMO-LUMO energy gap of L1 was calculated to be 2.65 eV, much lower than that of L1-CN (2.89 eV). This building-up energy gap is responsible for the blue shift of the UV–vis of L1. The experimental phenomenon was well in line with the theoretical results. Therefore, we can summarily conclude that L1 detected CN- by a nucleophilic addition reaction.
Conclusions
In summary, we have reported an effective technique for the construction of a highly selective furfural derivative–indolium probe, called L1, to detect cyanide anion in DMSO/H2O (1:1, v/v) solution. The L1 probe selectively detected CN- through color change and through UV-vis and fluorescence spectroscopic data. Visual observation revealed that the solution color changed from yellow to lime. Also, the color change was followed by yellow fluorescence quenching. The mechanism for detection of CN- by the L1 probe was characterized as a nucleophilic addition reaction, as determined by 1H NMR, MS, and DFT measurements. The L1 probe exhibits high selectivity for CN-, even in the presence of other anions, and has a low limit of detection (LOD) of 3.65 × 10-7 mol/L, demonstrating that the L1 probe is an effective and convenient sensor for recognizing cyanide in complicated wastewater systems.
FIGURE 4: (a) LC–MS spectrum of L1 upon addition of CN- (CH CN). (b) LC–MS 3 spectrum of L1 (CH3CN). (c) Suggested mechanism for the L1 to CN . Partial H NMR spectra of L1 in the presence of different equivalents of CN-.
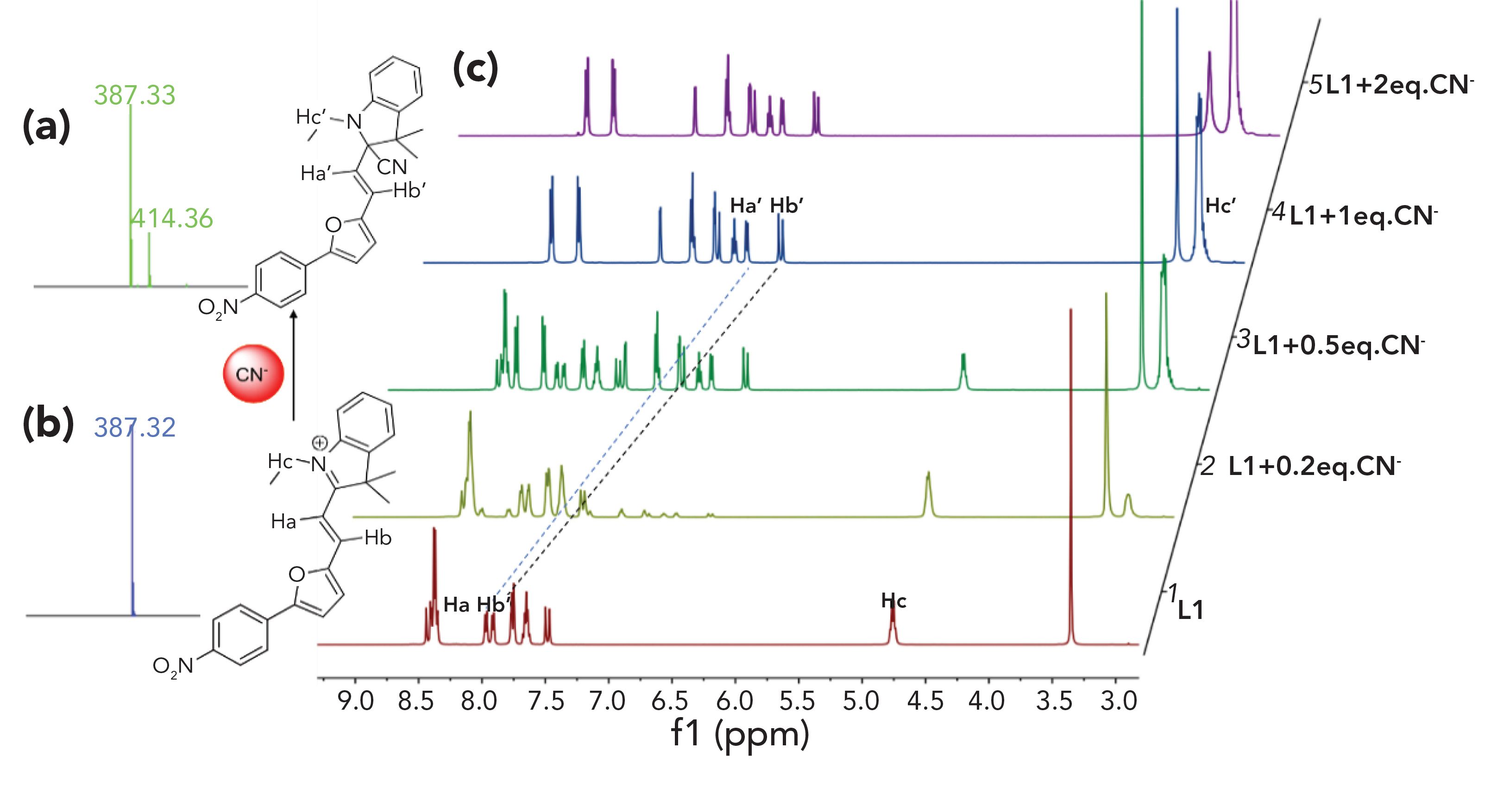
FIGURE 5: (a) Frontier molecular orbitals of L1 and L1-CN obtained from the DFT calculations using Gaussian 09 program. (b) The optimized ground-state structures of L1 and L1-CN.
Acknowledgment
This study was supported by the scientific research foundation of Hainan University (KYQD[ZR]1917) and the Hainan Provincial Natural Science Foundation of China (319QN165).
References
(1) X. Chen, T. Pradhan, F. Wang, J.S. Kim, and J. Yoon, Chem. Rev. 112, 1910–1956 (2012).
(2) X. Li, X. Gao, W. Shi, and H. Ma, Chem. Rev. 114, 590–659 (2014).
(3) Y. Zhou, J. F. Zhang, and J. Yoon, Chem. Rev. 114, 5511–5571 (2014).
(4) C.-B. Bai et al., ACS Omega 5, 2488– 2494 (2020).
(5) C.-B. Bai et al., Ind. Eng. Chem. Res. 59, 8125–8135 (2020).
(6) L. G. Nandi, C. R. Nicoleti, I. C. Bellettini, and V. G. Machado, Anal. Chem. 86, 4653–4656 (2014).
(7) X. Huang, X. Gu, G. Zhang, and D. Zhang, Chem. Commun. 48, 12195– 12197 (2012).
(8) L. Peng, M. Wang, G. Zhang, D. Zhang, and D. Zhu, Org. Llett. 11, 1943–1946 (2009).
(9) D.S. Kim, Y.-M. Chung, M. Jun, and K. H. Ahn, J. Org. Chem. 74, 4849–4854 (2009).
(10) Z. Xu, X. Chen, H. N. Kim, and J. Yoon, Chem. Soc. Rev. 39, 127–137 (2010).
(11) H. Tavallali, G. Deilamy-Rad, A. Parhami, and N. Hasanli, J. Hazard. Mater. 266, 189–197 (2014).
(12) S. Liu, M. Yang, Y. Liu, H. Chen, and H. Li, J. Hazard. Mater. 344, 875–882 (2018).
(13) R. Koenig, Science 287, 1737–1738 (2000).
(14) F. Wang, L. Wang, X. Chen, and J. Yoon, Chem. Soc. Rev. 43, 4312–4324 (2014).
(15) S. Goswami et al., Tetrahedron Lett. 54, 1785–1789 (2013).
(16) G. Ding, H. Zhou, J. Xu, and X. Lu, Chem. Commun. 50, 655–657 (2014).
(17) J.P. Anzenbacher, D.S. Tyson, K. Jursíková, and F.N. Castellano, J. Am. Chem. Soc. 124, 6232–6233 (2002).
(18) P.X. Pei, J.H. Hu, C. Long, and P.W. Ni, Spectrochim. Acta, Part A. 198, 182–187 (2018).
(19) L. Yang, X. Li, J. Yang, Y. Qu, and J. Hua, ACS Appl. Mater. Interfaces 5, 1317–1326 (2013).
(20) X. Zheng et al., ACS Appl. Mater. Interfaces 6, 7996–8000 (2014).
(21) X. Lou, L. Qiang, J. Qin, and Z. Li, ACS Appl. Mater. Interfaces 1, 2529–2535 (2009).
(22) J. Li et al., Sens. Actuators, B. 220, 986–991 (2015).
(23) L. Yuan, W. Lin, and Y. Yang, Chem. Commun. 47, 6275–6277 (2011).
(24) P. Zhang et al., Dyes Pigm. 99, 857–862 (2013).
(25) W.T. Gong, Q.L. Zhang, L. Shang, B. Gao, and G.L. Ning, Sens. Actuators B. 177, 322–326 (2013).
(26) W. Xu et al., Sens. Actuators B. 251, 366–373 (2017).
(27) M. Yoo, S. Park, and H.J. Kim, Sens. Actuators B. 220, 788–793 (2015).
(28) G. R. You et al., Sens. Actuators B. 202, 645–655 (2014).
(29) X. Cheng et al., ACS Appl. Mater. Interfaces 4, 4387–4392 (2012).
(30) M. R. Ajayakumar et al., ACS Appl. Mater. Interfaces 5, 6996–7000 (2013).
(31) W.C. Lin, S.K.Fang, J.W. Hu, H.Y. Tsai, and K. Y. Chen, Anal. Chem. 86, 4648–4652 (2014).
(32) L. Wang, L. Li, and D. Cao, Sens. Actuators B. 239, 1307–1317 (2017).
(33) S. Das et al., RSC Adv. 4, 9656–9659 (2014).
(34) Q. Lin, Y.P. Fu, P. Chen, T.B. Wei, Y.M. Zhang, Tetrahedron Lett. 54, 5031–5034 (2013).
(35) A. K. Mahapatra et al., Chem. Asian J. 9, 3623–3632 (2014).
Zengyan Chang, Deng Pan, Xinyu Fan, Shi Tang, Junjian Li, and Ai-Qun Jia are with the Key Laboratory of Tropical Biological Resources of the Ministry Education, at the School of Life and Pharmaceutical Sciences, at Hainan University, in Haikou, in the People's Republic of China. Direct correspondence to: lijunjian1020@163.com

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.
Tracking Molecular Transport in Chromatographic Particles with Single-Molecule Fluorescence Imaging
May 18th 2012An interview with Justin Cooper, winner of a 2011 FACSS Innovation Award. Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 ? the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
Can Fluorescence Spectroscopy Evaluate Soil Dissolved Organic Matter Dynamics?
February 20th 2025A new study published in Chemical Engineering Journal by researchers from Northeast Agricultural University in China reveals that biochar aging, influenced by environmental factors like UV exposure and wet-dry cycles, alters dissolved organic matter composition and affects its effectiveness in remediating cadmium-contaminated soil.
New Fluorescent Raman Technique Enhances Detection of Microplastics in Seawater
November 19th 2024A novel method using fluorescence labeling and differential Raman spectroscopy claims to offer a more efficient, accurate approach to detect microplastics in seawater. Developed by researchers at the Ocean University of China, this method improves both the speed and precision of microplastic identification, addressing a key environmental issue affecting marine ecosystems.