Advances in Spectroscopy for Detection and Identification of Potential Bioterror Agents
Special Issues
More-specific, sensitive, rapid, and portable spectroscopic methods detect biological threats.
Pathogens, such as bacteria and viruses, and the toxins produced by some organisms are viewed as potentially easier weapons of mass destruction for terrorist groups and rogue governments to develop, and as such, require particular vigilance in detection and identification on the part of the world community. Spectroscopic methods have been used for many years to amplify the signal provided by primarily nucleic acid–based and antibody-based detection regimes; these continue to be improved and refined, alongside newer attempts to develop spectroscopic techniques that can be used in the absence of specific molecular interactions. For example, mass spectrometry currently is being applied in proteomic-type methods to identify pathogenic bacteria from groups of proteins within them that possess fragments of unique masses; in effect, a pathogen-specific mass signature. Conjugation of gold nanoparticles and fluorescent reporter groups to specific antibodies or nucleic acids that reliably bind to unique partners, characteristic of a particular pathogen or toxin, allows a variety of spectroscopic techniques to amplify the signal arising from ever smaller numbers of fluorophores or associating nanoparticles. Among these spectroscopic methods are a number exploiting surface plasmon resonance, two-photon Rayleigh scattering, surface-enhanced Raman spectroscopy, and probe-dependent fluorescence quenching. Electrochemical methods for detecting specific reporter–substrate interactions also are being exploited for their sensitivity. Here, the recent research literature is reviewed to identify representative advances in the use of spectroscopy to improve detection capabilities for pathogens and toxins potentially used as bioweapons, with respect to specificity, sensitivity, rapidity, and portability to the field.
There is growing concern today among scientists, policymakers, and the general public that pathogenic microbes, such as bacteria and viruses, and toxins generated by a variety of organisms might be converted into biological weapons by terrorist groups or rogue governments (1). The primary motivating factors for this concern about the possibility of "weaponizing" a pathogen or toxin are the ready availability of the relevant microorganisms, the limited need for resources to effect the transformation of a pathogen into a weapon (all of which would be available to interested governments and organizations for a price most could manage), and the ease of transmission of the biological agent into and among the target population (2). Though some commentators have suggested that the interest among radical groups and governments in these weapons is considerably less than generally supposed (3,4), the ease of deployment of such a bioagent dictates an aggressive defense (5). Making pathogens and organisms that synthesize toxins unavailable, or severely restricting their dissemination to prevent their misuse, is simply not feasible: even Bacillus anthracis, the causative bacterium in anthrax, is widespread in the soil where livestock are raised (6). Clearly, the key to protecting human populations from biothreats used maliciously lies in rapid detection and identification of the dangerous agent, both before and during a bioterror attack. Improvements in detecting and identifying such bioagents most likely will arise from advances in detecting smaller amounts of the relevant material more rapidly and reliably, minimizing sample preparation before evaluation, and making detection and identification technology portable to any environment where needed.
Spectroscopy is a key tool in improving methods for detecting and identifying bioterror agents, as most key molecules constituting such agents are of sufficient complexity that they have identifiable spectroscopic features and give strong signals. Currently, most instrumental techniques for generally detecting and identifying microbes, toxins, and biothreat agents constituting a smaller group within that larger classification scheme rely on one of two methods for recognizing a specific molecular entity:
- Antibodies which bind specifically to a defining molecular group or set of groups on the surface of the agent, or
- Nucleic acids of specific sequences that pair complementarily to particular sequences in the nucleic-acid population (typically the genome) of the agent organism.
Both of these specifically binding entities are highly engineered by nature to recognize their targets with extremely high specificity and affinity, even among much more numerous competing molecules of similar structure.
Detecting the formation of the specific complex, especially at low concentrations, is where spectroscopic methods traditionally have made their greatest contributions to this specific detection and identification effort. Attachment of a fluorescent label or a uniquely labeled nanoparticle to the antibody or reporter nucleic acid makes the antibody's or nucleic acid's behavior accessible to a variety of spectroscopic methods, but more recently, experimenters have been developing techniques to exploit the spectroscopically active groups on the target molecules themselves, such as the toxin of interest or a cell-surface or virus-surface carbohydrate or protein. Others have adapted established reporter-based techniques to more compact and portable instrumentation, or have broadened the applicability of a method to more complex and less purified samples, permitting more rapid determinations with less sample preparation.
Fluorescent Detection of Antibody-Based Reporter Groups
Because of the high specificity of a single antibody for its epitope (the group on the target antigen to which the antibody binds), a wide variety of methods for identifying and quantifying specific molecules have been based on antibodies over the last several decades. Many of these methods can be extended to viruses and bacteria (or other types of cells, such as fungi or cancer cells) if the molecule of interest is located on the surface of the larger entity. Warner and colleagues (7) have attached a quantum-dot reporter group to antibodies directed against the A-type botulinum toxin (produced by the bacterium Clostridium botulinum, the causative organism in the food-poisoning syndrome botulism), a protein of common concern as a potential bioterrorism agent. These investigators used the quantum dot in the traditional "sandwich"-type immunoassay (Figure 1), in which a "capture" antibody is bound to a solid surface and then used to hold the antigen (botulinum toxin) while other molecules are washed away. A second antibody, for detection, which also binds to the antigen of interest and bears the fluorescent reporter group, is incubated then with the solid support and forms an antibody "sandwich" with the antigen in between. After excess detection antibody is washed away, the quantum dot fluoresces (at 655 nm) under activation by light at the excitation wavelength (365 nm). The quantum dot gives both greater sensitivity, allowing detection in the low picomolar range, and more stable emission signal, because of the lack of photobleaching seen with an earlier reporter group.
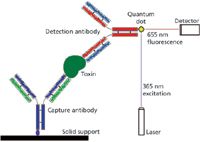
Figure 1: The quantum-dot-fluorescence, "sandwich"-type immunoassay of Warner and colleagues (7). The toxin molecule being assayed (heart-shaped, dark green) is "sandwiched" between the capture (dark blue and green) and detection (light blue and red) antibodies. Those capture antibodies not binding a toxin molecule do not retain any detection antibodies after the final wash is performed, so the quantum dot (yellow diamond) fluoresces in proportion to the bound detection antibody, and thus in proportion to the bound toxin, when excited by laser light of the appropriate wavelength. The capture antibody is bound to the solid support, anchoring the entire complex throughout the washes and the spectroscopic analysis, by a short linker (purple oval).
Fluorescent Detection of DNA Amplified by Polymerase Chain Reaction
Since the development of the polymerase chain reaction (PCR) technique two decades ago for the amplification of very small amounts of DNA, visualization and quantification of the DNA produced has depended most commonly on the fluorescence of a reporter group indicating the quantity of the desired DNA present. In recent years, real-time PCR has used instruments with built-in fluorescent optics and continually more clever ways to employ fluorescent reporter groups to reflect as accurately as possible the presence and quantity of the desired DNA molecule. Kutyavin (8) recently reported a technique he describes as "snake" (because of the shape of the hybridized DNA intermediate formed), in which an oligonucleotide probe designed to basepair exclusively with an interior segment of the target DNA is degraded by the polymerase's 5'→3'-nuclease activity, separating the attached fluorophore from its neighboring quenching agent and allowing the fluorophore to fluoresce in proportion to its free concentration (Figure 2). The "snake" modification improves the signal-to-noise ratio of the fluorescence monitoring by keeping the fluorescent probe able to bind the target DNA independently of the portion of the DNA being synthesized, rather than becoming incorporated into the synthesized strand. The author did not test the method specifically on DNA segments associated with bioterrorism threats, but the method is applicable, in principle, to any pathogenic organism, such as the bacteria giving rise to anthrax, bubonic plague, staphylococcal infections, Legionnaire's disease, and a host of other illnesses. The primary advantage of PCR in detecting microorganisms is that it requires little sample and almost no sample preparation (the small sample size allows most contaminants simply to be diluted out in constituting the reaction).
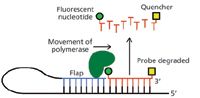
Figure 2: "Snake" real-time polymerase chain reaction assay, monitored by fluorescence. In the work of Kutyavin (8), a primer used for amplification (complementary to the light blue 3'-end of the DNA strand shown here) is synthesized with a 5'-extension containing the same sequence as that to which the "flap" (light blue segment) pairs. This allows the "snake" secondary structure to form (black and light blue DNA strand). The fluorescent probe DNA, bearing a quenching group (yellow square) at its 3' end, basepairs with the segment immediately downstream of where the flap hybridizes, which allows the polymerase's (dark green, heart-shaped entity) 5'â3' nuclease activity to degrade the probe. This degradation of the probe releases the nucleotide bearing the fluorescent label (green circle) from the proximity of the quencher, permitting a fluorescence signal in quantitative agreement with the concentration of DNA synthesized (and, therefore, bearing the flap sequence).
Use of Nanoparticles to Detect Pathogenic Bacteria
Two-photon Rayleigh scattering: Singh and colleagues (9) used the two-photon Rayleigh scattering properties of gold nanorods (~100 nm in length, aspect ratio 2.7) to develop a sensitive method of detecting specific bacteria at low concentrations. The investigators conjugated a monoclonal antibody to the nanorods, directed against a cell-surface antigen unique to E. coli O157:H7, a strain often implicated in food poisoning outbreaks. Even at low concentrations of bacterial cells (50 viable cells per milliliter), enough nanorods aggregate to provide a reproducibly detectable two-photon scattering intensity, distinguishable from the background associated with free nanorod-conjugated antibodies or another strain of E. coli at significantly higher concentration. The spectral technique owes its aggregation-dependent sensitivity to the surface-plasmon excitation properties of the gold nanorods; one photon arising from the longitudinal electronic disturbance, and the other from the transverse effect, with the large aspect ratio contributing the substantial difference in wavelength between the two photons. A significant advantage of this type of technique is that it operates on intact bacterial cells, eliminating the need for disruption of the cells to make the antibody target accessible, as is the case with many nucleic-acid-based probes. It also avoids the need for additional antibodies of other specificities for detection, as in the widespread "sandwich" assays (the target molecule or particle "sandwiched" between the capture antibody, which binds it to the solid surface, and the detection antibody, which carries the signal-amplifying label, as described above and in Figure 1).
Direct Identification of Bioterror Agents by MS
During the past couple of decades, mass spectrometry (MS) has evolved to handle continually larger molecules, including proteins and complex carbohydrates. In the interest of pathogen identification, typically that of bacteria, some investigators have turned to profiling whole cells using the method, and matching the extremely complicated spectra that result, to libraries of such spectra for known microorganisms. Christner and colleagues (10) have recently used matrix-assisted laser desorption–ionization, time-of-flight (MALDI-TOF) MS to "fingerprint" a variety of blood-borne bacterial pathogens and test the limitations of the technique. They were able to identify the organism correctly in 87% of the 277 cases they attempted, with the failures largely arising from excessively small numbers of cells in those samples. The investigators also determined that Gram-positive bacteria, such as Strepto- and Staphylococci, were more likely to fail to be identified than Gram-negative bacteria, such as E. coli and Salmonella species. The development of reliable methods for identifying intact cells will be a significant step forward in bioterror-agent detection, as much less sample preparation will be required than for the analysis of individual components of those cells. MS is particularly well suited to this application because of the extremely high resolution and lack of overlapping signals it exhibits in contrast to other spectroscopic techniques.
Although whole-cell, MS-based analysis of a microorganism permits identification of that organism, the individual peaks in the spectrum often are not correlated to the identities of the individual molecules giving rise to them. A common use of MS to characterize pathogens is to fractionate the purified pathogen into individual molecular components, typically proteins, and then to identify each component separately using the spectral technique. Kong and colleagues (11) recently separated the constituent proteins of infectious bronchitis virus (IBV), a coronavirus infecting chickens (the virus responsible for the sudden acute respiratory syndrome [SARS] epidemic in Asia in 2003 was also a coronavirus), by two-dimensional gel electrophoresis, and then identified the individual proteins by digesting them with trypsin and subjecting them to MS. Additionally, the investigators found a large number of host-cell proteins packaged in the virus particles, identified by spectrum-matching to a library of proteins. Although the technique used here may shed light on the structure of the virus and vulnerabilities that can be exploited in confronting it clinically, the requirement to purify the virus and isolate it in significant quantities is a drawback to applying this method to identifying the virus in clinical samples. Viruses typically are present clinically in extremely low quantities, and require antibody- or PCR-based detection methods. However, the technique described by Kong and colleagues would be appropriate for identifying an unknown substance confiscated or discovered by authorities and thought to be a purified virus intended for use in a bioterrorism attack.
Direct Identification of Bioterror Agents by Vibrational Spectroscopy
Vibrational techniques, such as infrared and Raman spectroscopies, incorporate an enormous amount of structural information from their subject molecules. However, each suffers from a disadvantage: Infrared signals are swamped by the spectral contributions of water, which occurs in large quantities in biological samples, while the Raman technique shows extremely weak signals because of the small number of photons scattered. Driskell and colleagues (12) recently applied surface-enhanced Raman scattering (SERS) to the identification of a variety of rotaviruses, in which the isolated virus was deposited on an array of silver nanorods, such that laser excitation of the mobile electrons in the silver atoms enhanced the Raman peaks because of the virus particles by several orders of magnitude. The spectra for the individual virus species then were characterized by principal-component analysis, to obtain a reproducible spectral profile for each species against which unknown samples could be matched. Because of the intense signal enhancement resulting from the metal surface, the virus preparation did not require concentration, and cell lysates could be diluted somewhat before application to the silver substrate. Thus the sensitivity of the technique likely is sufficient to analyze bodily fluid samples from infected patients, but perhaps not yet high enough to detect virus reliably in those who are still presymptomatic.
Use of Surface Plasmon Resonance to Characterize Toxin Binding
Flagler and colleagues (13) recently used surface plasmon resonance to compare the binding preferences of Shiga toxins 1 and 2, which are often mentioned as potential bioterror agents, toward the cell-surface carbohydrates they exploit to enter cells and exert their toxicities. The results of the surface plasmon resonance binding determination were qualitatively similar to those obtained using an enzyme-linked immunosorbent assay (ELISA — an antibody-based detection method similar to the "sandwich" assay described above and in Figure 1), but the ELISA was more sensitive by a couple of orders of magnitude. The conditions under which the surface plasmon resonance experiment was conducted may be possible to refine further to improve the method's sensitivity. However, it is also possible that the continuous flow of the toxin-containing solution, compared to the lack of agitation in the ELISA, plays a much greater role in destabilizing the complex, and that surface plasmon resonance is simply not the ideal choice for studying this specific toxin–receptor complex.
Electrochemical Detection of Specific Molecules
Liu and colleagues (14) recently have published work in which they devised an electrochemical sensor for the presence of interferon-γ (gamma) that could, in theory, be modified to detect the presence of a toxic biothreat agent, such as botulinum toxin or ricin. The authors use an aptamer, which is a nucleic-acid molecule that folds into a specific conformation capable of binding a target molecule with substantial specificity. Here they employ a 34-nucleotide DNA segment, that, absent interferon-γ, forms a stem-and-loop structure, bringing the redox-labeled 3'-end in proximity to the 5'-end, which is bonded to a gold surface serving as an electrode (Figure 3). When interferon-γ binds to the aptamer, it disrupts the stem-and-loop structure, drawing the redox label away from the gold surface and impeding electron transfer. The extent of the impedance is quantified by square-wave voltammetry, and the authors determine that it is in proportion to the amount of interferon-γ present and shows high sensitivity (ability to detect, reliably, quantities in the range observed in living organisms).
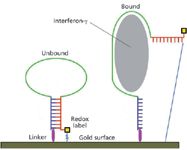
Figure 3: Electrochemical detection of interferon-γ by aptamer-based electrical impedance. According to Liu and colleagues (14), the binding of the subject molecule, in this case interferon-γ (gray oval), disrupts the basepairing (blue and red ladder) between the complementary segments of the aptamer (dark blue, green, and red structure). This draws the redox label (yellow square) out of proximity of the gold surface, the latter serving as an electrode, and the resulting distance (narrow blue line on far right) is too great to support electron transfer. The result is a measurable electrical impedance indicating the concentration of interferon-γ. The aptamer is linked to the gold surface by a short thiol-bearing group (magenta oval), permitting the subject molecule to be washed away and the redox label to be regenerated.
Conclusions
It is clear from current research in bioterror-agent detection that fluorescence spectroscopy is still the major tool by which specific target molecules are quantified, and that trapping these molecules is still done, for the most part, using antibodies or amplifying them using PCR. However, because of continually increasing sensitivity in MS, the direct detection of pathogen by-products and whole cells by that class of methods is growing rapidly. Other spectroscopic methods, including those of electrochemistry, are finding utility in detection schemes as they can be applied to the recognition of specific features on target molecules, or conjugated to other molecules that specifically recognize the targets. The methods and examples cited here are not necessarily the most appropriate among the many others, which were not mentioned, but are representative of the current trends toward more specific, sensitive, rapid, and portable means to detect biological threats of a wide variety.
References
(1) M.J. Selgelid, J. Med. Ethics 30(6), 558–560 (2004).
(2) R.G. Evans and S.J. Lawrence, Disease Manag. Health Outc. 14(5), 265–274 (2006).
(3) Y. Sharan, "The Bioterrorism Threat," in Risk Assessment and Risk Communication Strategies in Bioterrorism Preparedness (NATO Security Through Science Series A: Chemistry and Biology), M. Green et al., Eds. (Springer, Dordrecht, The Netherlands, 2007), pp. 47–54.
(4) L.A. Meyerson and J.K. Reaser, Front. Ecol. Environ. 1(6), 307–314 (2003).
(5) J.M. Nolan et al., Biosecur. Bioterror. 8(4), 365–372 (2010).
(6) I.L. Pepper and T.J. Gentry, Soil Sci. 167(10), 627–635 (2002).
(7) M.G. Warner et al., Biosens. Bioelectron. 25(1), 179–184 (2009).
(8) I.V. Kutyavin, Nucl. Acids Res. 38(5), e29 (2010).
(9) A.K. Singh et al., ACS Nano 3(7), 1906–1912 (2009).
(10) M. Christner et al., J. Clin. Microbiol. 48(5), 1584–1591 (2010).
(11) Q. Kong et al., Proteome Sci. 8(1), 29 (2010).
(12) J.D. Driskell et al., PloS One 5(4), e10222 (2010).
(13) M.J. Flagler et al., Biochemistry 49(8), 1649–1657 (2010).
(14) Y. Liu, Anal. Chem. 82(19), 8131–8136 (2010).
Eric W. Fisher is with Protein Molecules, Inc., Springfield, Illinois.

Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.