Mid-Infrared Vibrational Spectroscopy Standoff Detection of Highly Energetic Materials: New Developments
A review of recent developments in MIR spectroscopy that have potential for field deployment in defense and homeland security applications for standoff detection of highly energetic materials (HEM) and homemade explosives (HME), as well as in forensic sciences applications.
Vibrational spectroscopy practiced in the mid portion of the infrared spectrum (MIR): 400–4000 cm-1 (25–2.5 μm) provides the basis for development of several of the most powerful methods of qualitative and quantitative chemical analysis. In recent years, technological advances in optical fibers and optics in the MIR used to couple probes, microscopes, and telescopes have resulted in remote sensing capabilities that span the near field under a microscope objective to the far field using reflective IR telescopes. In this article, we review recent developments in MIR spectroscopy that have the potential for field deployment in defense and homeland security applications for standoff detection of highly energetic materials (HEM) and homemade explosives (HME), as well as in forensic sciences applications.
Infrared (IR) spectroscopy has played an important role in the characterization of highly energetic materials. IR spectroscopy contributions in the area of laboratory-based detection and characterization of highly energetic materials (HEMs) were reviewed by Steinfeld and Wormhoudt (1) in 1998 and later updated by Moore in 2004 (2) and 2007 (3). As discussed in these reviews, solid and liquid phase IR reference spectra of HEMs and propellants (1) and cyclic organic peroxides (4) are available for characterization and identification purposes. Remote gas phase sensing has been limited to high vapor pressure chemical agents, toxic industrial compounds (TICs), and high-vapor-pressure cyclic organic peroxides (for example, TATP) (5,6). Most HEM contain nitro (–NO2) groups, whose vibrational signatures are very characteristic. Beal and Brill (7) provided a comprehensive treatise on the behavior of the vibrational spectra of the -NO2 groups in more than 50 energetic compounds. They noted that the scissor vibrations (842–846 cm-1) are remarkably insensitive to differences in the local chemical environment and therefore could be used as detection tags. Similarly, the presence of nitro group bands evidenced by signatures at 760 cm-1, ~1350 cm-1 (symmetric NO2 stretch), and ~1600 cm-1 (asymmetric NO2 stretch) can be used as markers within nitro compounds. These signatures can be used universally to detect these compounds from a distance. The merits and challenges for such a technique include
- Remote: the technique should be capable of detecting HEM and HEM at long distances (that is, tens to hundreds of meters).
- Universal: the technique should detect all HEM–HME compounds.
- Specific: the technique should be able to identify HME–HEM with relatively few false positives; this requirement suggests the use of spectroscopy.
- Rapid: the technique should be capable of providing results within a very short time.
- Sensitive: the technique should be capable of detecting HME–HME compounds with sufficient sensitivity.
Samples and Standards Preparation for Traces of HEM–HME on Substrates
Two methods were developed for the preparation of homogeneous samples and standards of solids and traces deposited on surfaces: sample smearing (Figure 1a) and thermal inkjet deposition (Figure 1b). Specially prepared samples were used in experiments that required fine control of the analyte distribution on the surface. Importantly, these methods can be used to establish reliable standards that can support an instrument response validation program.
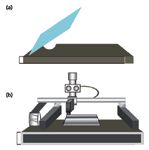
Figure 1: Methods for transferring a solid sample onto substrates: (a) sample smearing; (b) TIJ deposition.
Smearing and thermal inkjet sample deposition have proven to be good methods for transferring explosives onto stainless steel, plastic, and glass surfaces (8–15). Sample smearing preparation generally adheres to the preparation of a standard solution in a solvent in which the test chemical is highly soluble. Thermal inkjet technology offers several advantages compared to smearing deposition. This method is not subject to human error and provides more uniform coverage. Moreover, the surface loading concentration can be varied by changing the number of passes delivered of the sample, the dispensing frequency, and the applied energy and pen architecture. In addition, this method features precise delivery of a number of droplets with well-characterized mass and concentration. Only one solution is required, avoiding dilutions that can increase the analytical errors introduced by human involvement. HEM solutions were placed into the inkjet cartridge and the back pressure was set to 3 in. of water using an external back pressure controller. The solutions were dispensed on stainless steel plates using zero dot spacing script (space between drops using an HP inkjet) at a printing resolution of 600 dots per inch (dpi). After the solvent evaporated, the spectra of the samples were collected under the same conditions as for the substrate plates (blanks).
Chemometrics Detection, Identification, and Discrimination
Partial least squares (PLS) chemometrics routines were used to build robust cross calibration models of predicted versus true surface loadings (16). PLS is a quantitative spectral decomposition technique that is related closely to principal component regression (PCR). Instead of decomposing the spectral matrix into a set of eigenvectors and scores first and then regressing them against the concentrations as a separate step, PLS uses the concentration information during the decomposition process. This weights spectra containing higher constituent concentrations more heavily than those with low concentrations. The primary aim of PLS is to place as much concentration information as possible into the first few loading vectors. PLS simply takes advantage of the correlation relationship that already exists between the spectral data and the constituent concentrations. In effect, this technique generates two sets of vectors and two sets of corresponding scores. One vector set includes the spectral data, and the other vector set includes the constituent concentrations. Presumably, the two sets of scores are related to each other through some type of regression, and a calibration model is constructed.
Fiber-Optic Coupled-Grazing Angle Probe FT-IR
Sensors can be constructed in the mid-infrared (MIR) using any of six basic sensing schemes: absorption, transmission, reflection, grazing angle (GA) reflection, attenuated total reflection (ATR), and a variant of the ATR effect known as the fiber evanescent wave (17,18). Reflection-absorption infrared spectroscopy (RAIRS), operating at the grazing angle, is an excellent optical absorption technique available for measuring low concentrations of chemical compounds adhered to reflective surfaces such as metals (8–15, 19–21). MIR spectroscopy, operating at the ''grazing angle'' of incidence (approximately 80° from the surface normal), is considered to be one of the most sensitive optical absorption techniques available for measuring low concentrations of chemical compounds deposited on surfaces such as metals, glasses, and plastics (19).
Fourier-transform reflection absorption infrared spectroscopy (FT-RAIRS), combined with a GA probe, has been used outside the sample compartment as a near-field sensor for the detection of organic peroxides (8) and nitro-based energetic materials on stainless steel, plastics, and glasses (9–12). The capability of the technology to characterize and detect mixtures has also been addressed (14,15). The attractive features of this technique include portability, a simple and rugged design, high sensitivity, and short analysis time. These features suggest the potential use of MIR-coupled FT-IR for energetic materials detection, characterization, and decontamination in national security and defense applications, including airport screening and military applications.
A clear disadvantage of fiber-optic coupled-grazing angle probe (FOC-GAP)-RAIRS is that this method is an open-path experiment in which the IR beam passes through the atmosphere before and after interacting with the sample. As a result, some absorption bands from water vapor (3800–3400 and 1600 cm-1) and CO2 (~2349 cm-1) are observed. The solution to this problem is to collect a single channel background spectrum before each sample run. Another possible solution is to mathematically eliminate these bands using algorithms that are incorporated in standard FT-IR data acquisition and analysis software packages. However, the nitro bands in nitrogen-based high explosives are very intense, often rendering these atmospheric bands negligible. NO2 asymmetric (~1600 cm-1) and symmetric (~1250–1375 cm-1) bands act as vibrational signatures for the discrimination of nitroaromatic HEMs (TNT, DNT, Tetryl), nitroaliphatic HEMs (PETN, HMX, RDX), nitramines (HMX, Tetryl), and nitrate esters (PETN). RAIRS spectra of HEM and HME are shown in Figure 2. The limits of detection of this technique for nitroaromatic HEMs are in μg/m2 (13,14).

Figure 2: Grazing angle spectra of HEMs: (a) Cyclic organic peroxide TATP (an HME): 20-, 40-, and 100-μg/cm2 surface loadings on stainless steel. (b) PETN, TNT, and PETNâTNT mix (Pentolite) deposited on a metallic substrate.
The ability to discriminate between neat energetic compounds and their mixtures also has been demonstrated using FT-IR technology. Detection and discrimination between a mixture and its constituent compounds for the well-known formulation of TNT and PETN (pentolite) were used as a proof of concept, as shown in Figure 2b (13). The experiments demonstrated that both explosives have signature bands that can be distinguished from the rest of the components present in the sample even when relatively low surface concentrations of the samples are present. The nitro stretching vibrations of both explosives appear at slightly different frequencies. Therefore, it can be clearly observed that the spectra of the pentolite mixtures contain features of both energetic materials and no significant band overlap.
A PLS regression algorithm (Quant2) that is incorporated in OPUS 4.2 software (Bruker Optics, Billerica, Massachusetts) was used to find the best correlation function between spectral and concentration matrices. A cross validation was performed, and the root mean square error of cross validation (RMSECV) and the root mean square error of estimation (RMSEE) were used as criteria to evaluate the quality of the method. Figure 3 illustrates the use of chemometrics-based routines to provide spectroscopic data enhancement. Figure 3a shows the improvement of the classification model that was constructed in terms of the percent correlation coefficient (%R2) as the number of scores or principal components (PC) used for modeling increases. Figure 3b shows the "leave-one-out" cross validation plot of predicted surface loading versus true surface loading for 2,6-DNT on stainless steel substrates using the Quant2 PLS routine of the OPUS 4.2 software.

Figure 3: (a) Improvement of the classification model (percent correlation coefficient, %R2) as the number of scores or principal components (PC) increases. (b) Predicted surface loadings versus true surface loadings for 2,6-DNT deposited on stainless steel substrates using "leave-one-out" cross validation by means of the PLS Quant2 routine.
Open-Path FT-IR
Open path interferometers were developed for atmospheric sensing of contaminant gases and vapors. Griffiths and collaborators (22) have recently revisited a 20-year-old application by applying chemometrics routines to define weak sample signals obscured by profuse atmospheric absorptions. Because of limitations, this very powerful technique had to wait for the birth of a new chemistry: the application of robust statistical techniques to spectroscopic data analysis and the possibility of coupling with linear discrimination functions to create an even more robust analytical tool. Open-path (OP)-FT-IR is a broad-area sensing technique that relies on spontaneous sample thermal emission (passive mode), but this technique can also be coupled to an active excitation source for pinpoint stimulated emissions from the sample.
We have found a new application for OP-FT-IR: detection of traces of HEM and HME deposited on surfaces (23,24). By coupling to a 6-in diameter reflective IR telescope, the field of view of the interferometer was narrowed to 10 mrad (0.57°), allowing measurements at distances up to 60 m. This system was used in passive mode for gas-phase detection of TATP vapors and in conjunction with an IR telescope-coupled MIR source for active-mode measurements of traces of HEM smeared on aluminum (Al) plates. For these experiments, 10 spectra were collected for each sample and 20 scans were collected of the background; all spectra were at 4 cm-1 resolution. Next, the technique was used to detect TNT traces deposited on Al substrates at a range of 4–30 m. During active mode sensing, both the source and collector were coupled to an IR telescope. Quantification studies on source-target distances for TNT surface loadings of up to 25 m (range) were achieved with high significance (24). Figure 4a shows remote IRS spectra obtained at fixed standoff distances of 1, 14.5, and 28 m for TNT surface loadings of 400 μg/cm2 on an Al substrate. A reference micro-FT-IR spectrum of TNT is also shown for comparison.

Figure 4: (a) SO IR spectra of Al plates with 400 μg/cm2 TNT at 1, 14.5, and 28 m. The micro-FT-IR spectrum of TNT is included as reference. (b) PLS cross validation plot at 30 m.
At source-target distances greater than 25–30 m, TNT signatures are very close to noise levels. However, using chemometrics and partial least squares (PLS) analysis, quantification of analyte loadings with statistically meaningful results is possible at distances of up to 30 m. Spectra were mean-centered, but no other pre-processing treatments were applied. In the cross validations shown in Figure 4b, true loading concentrations were plotted against predicted loading concentrations at 30-m range passive detection for TNT on Al substrates. By using an IR telescope-coupled MIR source, traces of TNT deposited as coatings on Al plates can be detected with high significance at standoff distances up to ~25–30 m. To detect HEMs on surfaces using this FT-IR technique, an open path between the source, the sample, and the detector are necessary. This situation is not always possible in real world scenarios. In the present case, atmospheric interferences (water vapor and CO2) did not present interference problems, and no special algorithms had to be applied.
Passive-mode OP-FT-IR detection of TNT on Al substrates produced even more impressive results. Temperature differences of 1–7 °C were studied in 1 °C increment. A tungsten lamp was placed on the back of the Al plates for sample heating, and standoff distances of 8–55 m were studied. In passive-mode experiments, careful alignment of the target and detector while recording spectra is not as critical. Figure 5a and 5b show the passive-mode spectra obtained for TNT, PETN, and their pentolite formulation.

Figure 5: Passive mode OP-FT-IR spectra. (a) TNT heated to 30â34 °C at 55 m range. (b) Spontaneous emissions of PETN, TNT, and pentolite at 32â36 °C at 20 m.
Most of the characteristic vibrational signatures of TNT were well defined, especially the bands that allow for the identification of TNT in the 700–1400 cm-1 spectral region. More importantly, the standoff spectrum agreed well with traditional infrared techniques: sample compartment, KBr pellet, micro-IR, attenuated total reflectance (ATR, both micro and macro), and grazing angle reflectance (GA, both micro and macro). Here, the vibrational band observed at 793 cm-1 was assigned to the C–CH3 stretch and the 2,4,6-NO2 scissors. Additionally, bands at 910 cm-1 (2,6-NO2 scissors and C–N stretch), 1087 cm-1 (C–H, ring in-plane bend), 1171 cm-1 (C–C, ring in-plane trigonal bend; 2,4,6-C–N and C–CH3 stretchings), and 1350 cm-1 (symmetric stretching vibration of the NO2 (nitro) group bond) were determined (25). In addition, all of the TNT signatures were observed to increase in intensity when the temperature of the target increased.
Quantum Cascade Laser Excitation in the MIR
The necessity of collimated and coherent light sources in the MIR is evidenced by the range limitation of the experiments described in the previous section. Even when coupled to IR telescopes to direct the light source on the substrates containing the target HEMs, the energy/area of the system described in the OP-FT-IR experiments would greatly benefit from a monochromatic, coherent, collimated, and polarized source: a laser. A quantum cascade laser (QCL), which operates in the MIR region, was first described by Faist and colleagues (26). The ability to tune a semiconductor laser has been demonstrated by several groups (26–30). The development of lasers with the ability to emit radiation in the 3–12 μm (833–3330 cm-1) spectroscopic range has advanced dramatically with the development of the quantum cascade (QC) and interband cascade (IC) lasers (27–28).
QCLs have revolutionized many areas of research and development in defense and security applications. Since the demonstration of continuous-wave operation of mid-infrared QCLs in 2002, the power levels of these laser systems have increased significantly (29,30). This advance has resulted in rapid development of QCL-based applications, such as photoacoustic spectroscopy (31–33), remote sensing (32,33), and the development of chemical sensors for various applications (34,35).

Figure 6: (a) QCL spectra RDX deposited on Al substrate at 110-, 150-, and 300-μg/cm2 surface loadings. (b) Reference micro-FTIR spectrum of TNT and the corresponding QCL excited reflectance spectrum.
We evaluated a widely tunable QCL scanner (1000–1600 cm-1) for detection and quantification of HEM deposited on reflective substrates. Figure 6 shows some of the results obtained for experiments using RDX and TNT. Other HEMs and HME deposited on Al, stainless steel, reflective glass, and other substrates of interest were also studied. Figure 6a shows that quantification studies are feasible using multivariable linear regressions, such as PLS QUANT2. Figure 6b shows that the important vibrational signatures of the HEM nitroaromatic TNT are well-preserved in the reflective QCL spectrum. The reference spectrum was acquired on a bench micro-FT-IR in reflectance mode. The main spectroscopic signatures of mixtures and formulations of HEMs are also preserved, as shown in Figure 7, in which a typical spectrum obtained for SEMTEX-H is compared to the spectrum of neat PETN.

Figure 7: QCL spectra of PETN, RDX, and SEMTEX-H formulation: 40.9% PETN and 41.2% RDX.
Outlook for Standoff MIR Detection of HEM–HME
The use of QCL in experiments with HEMs and HME promises a bright future for MIR standoff detection of these threat chemicals, which is of significant importance in national defense and security applications. Coverage of the entire 3–12 μm MIR range will require >2500 cm-1 of tuning range or wide band emissions. Thus, the development of broad-tuning QC gain media employed for such devices is imperative. With improvements, the present tuning ranges of ~300 cm-1 for standalone QCL systems are projected to achieve nearly 1000 cm-1 (28). QCL scanners have capabilities of ~600 cm-1, but these scanners are dispersively coupled to detectors for signal analysis and have a limited range in standoff detection. Coupling of QCL to reflective IR telescopes that would enable their use as long range excitation sources is under evaluation in several research laboratories and private companies. The results of such developments in QCL applications will enable the design of spectrometers that cover the 3–12 μm spectral range at >100 m standoff distances.
Acknowledgments
Part of the work presented in this study was supported by the U.S. Department of Defense University Research Initiative Multidisciplinary University Research Initiative (URI)-MURI Program under the grant number DAAD19-02-1-0257. The authors also acknowledge contributions from Mr. Aaron LaPointe from the Night Vision and Electronic Sensors Directorate, Fort Belvoir, Virginia; Department of Defense, Dr. Jennifer Becker, MURI Program Manager, Army Research Office, DoD, and Dr. Stephen J. Lee, Chief Scientist, Science and Technology, Office of the Director, Army Research Office/Army Research Laboratory, DoD.
Support from the U.S. Department of Homeland Security under Award Number 2008-ST-061-ED0001 is also acknowledged. However, the views and conclusions contained in this document are those of the authors and should not be considered a representation of the official policies, either expressed or implied, of the U.S. Department of Homeland Security.
References
(1) J.I. Steinfeld and J. Wormhoudt, Annu. Rev. Phys. Chem. 49, 203 (1998).
(2) D.S. Moore, Rev. Sci. Instrum. 75, 2499 (2004).
(3) D.S. Moore, Sensing and Imaging: An International Journal 8(1), 9 (2007).
(4) E.T. Urbansky, Crit. Rev. Anal. Chem. 30, 311 (2000).
(5) H. Lavoie, E. Puckrin, J.-M. Theriault, and F. Bouffard, Appl. Spec. 59(10), 11889 (2005).
(6) W.-J. Zheng, W.-Q. Jin, and J.-H. Su, Proc. SPIE Int. Soc. Opt. Eng. 6621, 66212A-1 (2007).
(7) R.W. Beal and T.B. Brill, App. Spec., 59, 1194-1202 (2005).
(8) O.M. Primera-Pedrozo, L.C. Pacheco-Londono, L.F. De la Torre-Quintana, S.P. Hernandez-Rivera, R.T. Chamberlain, and R.T. Lareau, Proc. SPIE Int. Soc. Opt. Eng. 5403, 237 (2004).
(9) O.M. Primera-Pedrozo, Y. Soto-Feliciano, L.C. Pacheco-Londoño, and S.P. Hernandez-Rivera, Sensing and Imaging: An International Journal. 10, 1 (2009).
(10) O.M. Primera-Pedrozo, N. Rodríguez, L.C. Pacheco-Londoño, and S.P. Hernández-Rivera, Proc. SPIE Int. Soc. Opt. Eng. 6542, 65423J (2007).
(11) Y. Soto-Feliciano, O.M. Primera-Pedrozo, L.C. Pacheco-Londoño, and S.P. Hernandez-Rivera, Proc. SPIE Int. Soc. Opt. Eng. 6201, 62012H (2006).
(12) O.M. Primera-Pedrozo, L.C. Pacheco-Londono, O. Ruiz, M. Ramírez, Y.M. Soto-Feliciano, L.F. De La Torre-Quintana, and S.P. Hernandez-Rivera, Proc. SPIE Int. Soc. Opt. Eng. 5778, 543–552 (2005).
(13) O.M. Primera-Pedrozo, Y. Soto-Feliciano, L.C. Pacheco-Londoño, and S.P. Hernandez-Rivera, Sensing and Imaging: An International Journal 9, 27 (2008).
(14) O.M. Primera-Pedrozo, Y.M. Soto-Feliciano, L.C. Pacheco-Londoño, and S.P. Hernández-Rivera, Sensing and Imaging: An International Journal 10(1), 1 (2009).
(15) M. Wrable, O.M. Primera-Pedrozo, L.C. Pacheco-Londoño, and S.P. Hernandez-Rivera, Sens. Imaging. 11, 147-169 (2010).
(16) K. Beebe, R. Pell, and M. Beth, Chemometrics: A Practical Guide (Wiley & Sons, New York, 1998).
(17) P.R. Griffiths and J.A. De Haseth, Fourier Transform Infrared Spectrometry, (Wiley, New York, 1986).
(18) J. Umemura, "Reflection–absorption spectroscopy of thin films on metallic substrates," in Handbook of vibrational spectroscopy, J.M. Chalmers and P.R. Griffiths, Eds. (Wiley & Sons, Chichester, U.K., 2002).
(19) P.J. Melling and P. Shelley, "Spectroscopic Accessory for Examining Films and Coatings on Solid Surfaces" U.S Patent 6,3,10,348, United States Patent and Trademark Office, Washington, DC.
(20) M.L. Hamilton, B.B. Perston, P.W. Harland, B.E. Williamson, M.A. Thomson, and P.J. Melling, Organic Process Research & Development 9, 337 (2005).
(21) B.B. Perston, M.L. Hamilton, B.E. Williamson, P.W. Harland, M.A. Thomson, and P.J. Melling, Anal. Chem. 79, 1231 (2007).
(22) P.R. Griffiths, L. Shao, and A.B. Leytem, Anal. Bioanal. Chem. 393, 45 (2009).
(23) L.C. Pacheco-Londoño, W. Ortiz-Rivera, O.M. Primera-Pedrozo, and S.P. Hernandez-Rivera, Anal. Bioanal. Chem. 395, 323 (2009).
(24) J. Castro-Suarez, L.C. Pacheco-Londoño, S.P. Hernández-Rivera, M. Diem, and M. Vélez, Standoff Detection of Explosives Using FTIR Telescope in "International Symposium on Spectral Sensing Research (ISSSR): Bridging the Gap from Emerging Technology to Warfighter," June 21–24, 2010, Springfield, Missouri.
(25) J. Clarkson, W.E. Smith, D.N. Batchelder, D.A. Smith, and A.M. Coats. J. Mol. Struct, 648, 203 (2003).
(26) J. Faist, F. Capasso, D.L. Sivco, C. Sirtori, A.L. Hutchinson, and A.Y. Cho, Science 264(5158), 553 (1994).
(27) P. Zorabedian, Tunable External Cavity Semiconductor Lasers; Tunable Lasers Handbook, F.J. Duarte, Ed. (Academic Press, San Diego, 1995).
(28) P.R. Buerki and M. Weida, Swept Sensor - A Novel Portable Battery-Operated Gas Sensing Solution, Doc. # D0268, Daylight Solutions, Inc., Poway, California, Nov. 2009.
(29) C. Gmachl, D.L. Sivco, R. Colombelli, F. Capasso, and A.Y. Cho, Nature 415, 883 (2002).
(30) M. Beck, D. Hofstetter, T. Aellen, J. Faist, U. Oesterle, M. Ilegems, E. Gini, and H. Melchior, Science 295, 301 (2002).
(31) Q. Wen and K.H. Michaelian, Opt. Lett. 33(16), 1875 (2008).
(32) A. Mukherjee, M. Prasanna, M. Lane, R. Go, I. Dunayevskiy, A. Tsekoun, and C.K.N. Patel, Appl. Opt. 47, 4884–4887 (2008).
(33) C.W. Van Neste, L.R. Senesac, and T. Thundat, Appl. Phys. Lett. 92, 234102 (2008).
(34) S. Wu, A. Deev, M. Haught, and Y. Tang, J. Chromatogr. A 1188(2), 327 (2008).
(35) C. Bauer, A.K. Sharma, U. Willer, J. Burgmeier, B. Braunschweig, W. Schade, S. Blaser, L. Hvozdara, A. Müller, and G. Holl, Appl. Phys. B. 92(3), 327 (2008).
Samuel P. Hernández-Rivera, John R. Castro-Suarez, Leonardo C. Pacheco-Londoño, Oliva M. Primera-Pedrozo, Nicolas Rey-Villamizar, and Miguel Vélez-Reyes are with the University of Puerto Rico-Mayaguez, Mayaguez, Puerto Rico. Max Diem is with Northeastern University, Boston, Massachusetts. The authors are part of the DHS Center of Excellence for Explosives Detection Mitigation and Response: ALERT at Northeastern University, Boston, Massachusetts, and University of Puerto Rico-Mayagüez, Mayagüez, Puerto Rico.

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.