Advantages of 1064-nm Portable Raman for Counterfeit Pharmaceuticals Authentication
A modern portable Raman instrument equipped with a 1064-nm laser is compared to one using a 785-nm laser for the identification of counterfeit pharmaceuticals.
The counterfeiting of pharmaceuticals is a global issue that is expanding each year. The severity of drugs counterfeited has increased from lifestyle drugs to include lifesaving (such as anticancer) types in recent times. The miniaturization of instrumentation has led to out-of-laboratory analysis for product authentication. Raman spectroscopy is ideal for pharmaceutical identifications and has been proven to detect counterfeit drugs. Portable instruments are available with dispersive technology and laser excitation at 785 nm for remote analysis. This study compares a modern portable Raman instrument equipped with a 1064-nm laser with one using a 785-nm laser. It is shown that authentic tablets are distinguished from the counterfeit tablets nondestructively, without the need to remove tablet coating.
Counterfeit medications are a problem globally, both in developing countries and developed nations. It is estimated that more than 10% of the global market is made up of counterfeits, with that number varying widely based on geographic region. Recently, there have been more reports of counterfeit lifesaving (for example, anticancer) types of drugs, not just the lifestyle drugs that made up much of the counterfeit market in the past (1,2). Fast, easy, and reliable methods to identify counterfeit drugs are crucial to reducing this problem. Raman spectroscopy is sensitive to molecular vibrations and as such is highly specific to the material that is measured. This makes Raman spectroscopy ideal for pharmaceutical identifications, and it has been proven to detect counterfeit drugs (3–6). The development of miniaturized portable Raman instruments in recent years has led to out-of-laboratory analysis for product authentication, which makes counterfeit identification practical at many different points in the supply chain and allows for faster detection and shut down of counterfeiting operations.
This study compares Raman spectra measured from two different portable Raman spectrometers: one with the more traditionally used 785-nm excitation and the other with the more recently developed 1064-nm excitation. It looks at the specificity of the Raman spectra from the different excitation wavelengths as well as the ability to measure nondestructively through tablet coatings and original medication packaging with no sample preparation.
Experimental
Samples were analyzed with two different instruments. Measurements obtained with 1064-nm laser excitation were done using a Rigaku Xantus-2 portable Raman instrument equipped with a cooled InGaAs detector. Measurements with the 785-nm laser excitation were performed using a portable Raman instrument equipped with a charge-coupled device (CCD) detector. For both instruments, a laser power of 300 mW was used.
Samples were measured in a variety of physical shapes: capsules, powders, tablets, and original vials containing powders. Samples that were capsules or powders were transferred to borosilicate vials and measured through glass vials using each instrument's specific vial holder. Tablets and powders in original vials were measured as is with each instrument's on-board point-and-shoot tip. Samples of authentic and counterfeit products used were: Alli (orlistat), Viagra (sildenafil citrate), and Duphaston (dydrogesterone). Authentic products were purchased from bona-fide pharmacies; counterfeits originated in Asia.
Raman data were reviewed post calibration from 300 to 1800 cm-1 using Thermo Omnic software (version 8.2), or with Igor Pro software (version 6.22A).
Results and Discussion
Comparison of 785-nm and 1064-nm Excitation
To obtain the best possible Raman spectra for the genuine samples, each of the authentic orlistat, sildenafil citrate, and dydrogesterone samples were measured with both the 785-nm and 1064-nm excitation instruments. Figure 1 shows the spectra for a genuine capsule of the weight loss medication orlistat collected with both 785-nm and 1064-nm excitation. The spectra are shown in the optimal comparative working range of 300–1800 cm-1. The spectrum collected with 785-nm excitation shows significant fluorescence as observed in the broad y-axis curvature. This fluorescence makes it difficult to see the sharper Raman peaks that are present on top of this curvature, and very significantly reduces the dynamic range available for the Raman signal. For many samples, fluorescence may be reduced by moving to a longer excitation wavelength. This is seen with the orlistat sample measured with 1064-nm excitation. The fluorescence is greatly reduced by moving to the 1064-nm excitation, thereby improving the Raman spectral quality and specificity, which makes the genuine sample easier to identify. Similar results were seen when authentic sildenafil citrate and dydrogesterone samples were measured with both the 785-nm and 1064-nm excitation instruments (data not shown).
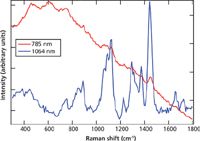
Figure 1: Genuine orlistat (Alli) Raman spectra collected with a 785-nm excitation instrument and a 1064-nm excitation instrument.
1064-nm Excitation for Distinguishing Authentic and Counterfeit Pharmaceuticals
The significance of the improved Raman spectral quality obtained with the 1064 nm excitation is evident by the comparison of the authentic product with the counterfeit. Authentic orlistat (60 mg) spectral data were overlaid directly with the counterfeit spectral data. The differences are clearly visible between the authentic and counterfeit samples measured with 1064-nm excitation, as shown in Figure 2. These differences can be seen without the need for further data treatment or chemometrics (such as principal components analysis).
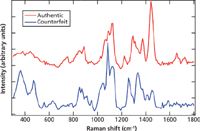
Figure 2: Authentic and counterfeit orlistat (Alli) capsule blend scanned using 1064-nm excitation, viewed with offset intensity.
Authentic and counterfeit versions of sildenafil citrate, which is used for the treatment of erectile dysfunction and pulmonary arterial hypertension, were also measured with 1064-nm excitation. These spectra are shown in Figure 3. The counterfeit and authentic versions of the sildenafil citrate are also easily distinguishable by sight. The differences between the genuine and the counterfeit can be seen at many different frequencies. In fact, the primary peaks that are similar between the two spectra are the triplet at low frequency which is characteristic of TiO2, a common ingredient in tablet coatings.

Figure 3: Offset spectra of an authentic 50-mg sildenafil citrate (Viagra) tablet and a counterfeit tablet measured with 1064-nm excitation.
To better understand the peaks that are present in the genuine sildenafil citrate spectrum measured with 1064-nm excitation, three different spectra were compared. The spectra of the coating for sildenafil citrate (Opadry Blue), an authentic 100-mg sildenafil citrate tablet measured through the coating, and an authentic 100-mg sildenafil citrate tablet with the coating mechanically removed are shown in Figure 4. Peaks specific to the Opadry Blue coating were seen around 399, 516, 635 (TiO2), 1460, and 1573 cm-1. Sharp, specific peaks for the active pharmaceutical ingredient (API), sildenafil citrate, were therefore confirmed at 1234 and 1525 cm-1 (7,8). These peaks are labeled with an asterisk in Figures 3 and 4. Of obvious interest with any counterfeit pharmaceutical is whether or not the API is present and in the proper amount. After identifying which peaks are caused by the sildenafil citrate, one can look back at Figure 3 where the peaks that are from the API are marked with an asterisk. Even by just looking at the results, it appears that the API is not present or at least is not present in as high a concentration in the counterfeit as in the genuine tablet.
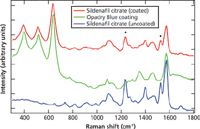
Figure 4: Sildenafil citrate coated and uncoated tablets (100 mg) and Opadry Blue coating material, offset spectra at 1064 nm.
A final comparison analysis for authentic and counterfeit samples was tested with the 10-mg dydrogesterone tablet. Dydrogesterone is used for the treatment of a wide variety of gynecological conditions related to progesterone deficiency. The genuine and counterfeit versions of dydrogesterone are shown in Figure 5. The differences between these two spectra are quite marked. The authentic type has a white Opadry coating, of which the counterfeit is devoid; the counterfeit shows no tell-tale sign of TiO2. Further analysis of the counterfeit dydrogesterone was done by comparing the measured spectrum to the high-resolution Aldrich Raman spectral library. This gave a strong match of the counterfeit tablet bulk contents to cellulose powder. The analysis of the Raman spectra for both the sildenafil citrate and dydrogesterone suggest the well-known fact that counterfeits are a significant health risk because many do not contain the API at all or not in the appropriate dosage amount.
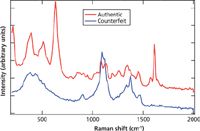
Figure 5: Authentic and counterfeit dydrogesterone, offset spectra measured with 1064-nm excitation.
1064-nm Raman Measurements Through Original Packaging
It is essential to understand the competency of Raman to identify samples through packaging, such as glass and plastic, so as to not expose the operator to a potent drug or contaminate the medication and to rapidly determine authenticity. Genuine dydrogesterone tablets were scanned either as a bare tablet or through the original blister packaging. Figure 6 shows the spectra of the two tablets measured with 1064-nm excitation. One was measured after removing it from the packaging and the second was measured through the blister pack. The spectrum measured through the blister pack used the Xantus-2 software to subtract a "user background" consisting of the Raman signature of an area of blister pack containing no product. It can be seen in the figure that these two spectra are nearly identical and that the genuine dydrogesterone could be identified through the blister pack.
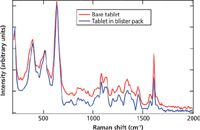
Figure 6: Overlay of dydrogesterone bare tablet and tablet scanned through blister pack with the packaging spectrum subtracted, measured with 1064-nm excitation.
An additional example of a nondestructive measurement through original packing was performed using amoxicillin powder packaged in a glass vial for injection. This is particularly relevant because of an alarming increase in incidents of counterfeited vial-containing-drug pharmaceutical preparations, for example, bevacizumab (Avastin) counterfeits were uncovered in the United Kingdom and the United States (1). Figure 7 shows spectra obtained for 1 g of amoxicillin in a vial for injection measured with both 785-nm and 1064-nm excitation instruments. From the spectra, it is clear that highly specific Raman peaks of the amoxicillin can be seen with the 1064-nm excitation instrument, but the 785-nm instrument only shows a broad fluorescent or optical phonon peak from the glass. Figure 7 also shows three replicate measurements of the amoxicillin sample collected on two days to assess the spectral reproducibility of the amoxicillin measured in the original packaging. As can be seen by the close overlap of the 1064-nm spectra, the reproducibility of measurements even through packaging is quite good.
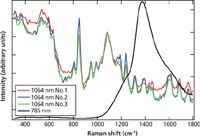
Figure 7: Amoxicillin analyzed through a glass vial with both 785-nm excitation and 1064-nm excitation instruments. Three replicate measurements were done with 1064-nm excitation.
Conclusions
Portable Raman spectroscopy, enabled by miniaturized instruments, continues to be at the forefront of the anticounterfeiting strategy. This study compares Raman spectra collected on a portable instrument with 1064-nm excitation to those collected on a 785-nm excitation instrument. In the cases shown, the 1064-nm excitation has a clear advantage in terms of reduced fluorescence and enhanced specificity, allowing for clear differences to be seen between Raman spectra from authentic and counterfeit medications. In addition to identifying the authentic medication, Raman spectroscopy can probe what is present (or not present, as is often the case with the API) in the counterfeit pharmaceuticals. Finally, it has been demonstrated that the 1064-nm excitation will allow for nondestructive Raman measurements of pharmaceuticals through their original packaging, which is essential for wide-scale authentication.
References
(1) U.S. Food and Drug Administration, "U.S. Food and Drug Administration. Avastin (bevacizumab): Counterfeit Product - FDA Issues Letters to 19 Medical Practices," Accessed online February 14, 2012: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm291968.htm.
(2) World Heath Organization, "Medicines: spurious/falsely-labelled/World Health Organization," Accessed online May 1, 2012: www.who.int/mediacentre/factsheets/fs275/en/index.html.
(3) F.M. Fernandez, M.D. Green, and P.N. Newton., Ind. Eng. Chem. Res. 47, 585–590 (2008).
(4) M. De Veij, P. Vandenabeele, K.A. Hall, F.M. Fernandez, M.D. Green, N.J. White, A.M. Dondorp, P.N. Newton, and L. Moens, J. Raman Spec. 38, 181–187 (2007).
(5) M. Hajjou, Y. Qin, S. Bradby, D. Bempong, and P. Lukulay, J. Pharma. Biomed. Anal. 74, 47–55 (2013).
(6) M.R. Witkowski, Am. Pharma. Rev. 8, 55–60 (2005).
(7) M. De Veij, A. Denker, P. Vandenabeele, D. de Kaste, and L. Moens, J. Pharm. Biomed. Anal. 46, 303–309 (2008).
(8) M. De Veij, P. Vandenabeele, and L. Moens, Spectrosc. Eur. 20, 7–10 (2008).
N.W. Broad is with NWBSpectroscopy.co.uk, Laboratory G310, in Sittingbourne, United Kingdom. C. Dentinger and J. Pasmore are with Rigaku Raman Technologies in Tucson, Arizona. Direct correspondence to: Claire.Dentinger@rigaku.com

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.