Analysis of Organometallic Compounds and Metallic Particles in Specialty Gases by Direct Injection Using Gas Exchange Device (GED) Coupled to Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
In this article, we introduce the concept of a gas exchange device (GED) and how it can be used to monitor organometallic compounds and metallic particles in specialty gases.
In this article, we introduce the concept of a gas exchange device (GED) and how it can be used to monitor organometallic compounds and metallic particles in specialty gases. The GED–inductively coupled plasma–mass spectrometry (GED–ICP-MS) workflow eliminates the need for any offline sample preparation, making it the ideal tool for online metallic impurities monitoring. This technology can be deployed to monitor specialty gas impurities in process chemicals at delivery, distribution points, and point of use.
Specialty gases for the semiconductor and electronics industries range from the pyrophoric or toxic gases required for thin-film deposition and doping processes (ammonia, silane, dichlorosilane, germane, diborane, arsine, and others) to the reactive and corrosive gases needed in different etching processes (chlorine, fluorine, halocarbons, nitrogen trifluoride, and more) (1). Conventionally used methods, such as impingers or filters, suffer from various limitations, including external contamination during the collection process, solubility of the organometallic compounds, metallic particles in impinger media, loss of metallic particles information, and filter material suitability. These limitations also do not include the labor-intensive and time-consuming sample preparation, as well as impinger unsuitability to be used for moisture sensitive gases (2).
A gas exchange device coupled with inductively coupled plasma–mass spectrometry (GED–ICP-MS) overcomes all of the abovementioned challenges. A GED–ICP-MS is a fully automated workflow that can be deployed in research and development (R&D) laboratories and in the fabrication (3). Two applications, carbon monoxide (CO) and hydrochloric gas (HCl), are discussed by comparing the results obtained by GED–ICP-MS versus the impinger (4,5).
Introduction
The semiconductor and electronics markets are constantly changing with the emergence of new technologies like artificial intelligence (AI), machine learning (ML), edge computing, 5G connectivity, and the “internet of things” (6). These trends pushed the industry to new barriers in node size, the advent of three-dimensional (3D) stacking, and the increasing complexity of chip designs requiring more precise, efficient, and environmentally friendly etching methods. Achieving such intricate manufacturing resulted in a shift from wet to dry etching with specialty gases at its core (7).
Specialty gases are used across the manufacturing process, and they can be classified into doping gases, epitaxial gases, ion implantation gases, photolithography gases, carrier gases, and more. Regardless of their application, specialty gases, manufacturers, transporters, and users have strict requirements for purity, composition, packaging, distribution, and storage (1).
Metallic impurities are commonly measured using a highly sensitive technique such as ICP-MS, which is capable of accurately quantifying ultra-trace levels of metallic contamination—dissolved or singular nanoparticulate (NP)—in chemicals used throughout integrated circuit (IC) manufacturing (8). However, gases are difficult to analyze directly by ICP-MS because even a small amount of these gases will extinguish the instrument argon (Ar) plasma. Complicating matters further is that the industry lacks the metallic gas standards required for accurate quantification. That said, specialty gases need to undergo sample preparation to be analyzed for metallic impurities.
Two sample preparation techniques are commonly used in the industry, the most common of which is the impinger method. This technique employs specially designed bubble tubes that flow a known volume of the sample gas into a liquid medium that will chemically or physically react with the chemical of interest. The liquid is then analyzed by ICP-MS, with the other employed technique being filtering. The sample gas flows through a series of filters that are then digested and analyzed by ICP-MS. In both approaches, sample preparation is tedious and time-consuming. Most importantly, it masks the chemical structure of the detected impurities, making it difficult to point out the source.
Gas direct injection via a GED–ICP-MS resolves those challenges while maintaining the metallic impurities structure for source identification. This technique enables direct sample gas analysis via injection into a GED—a pressured nanometer porous membrane—that exchanges the sample gas with argon while maintaining the organometallic compounds and metallic particles in its center flow, delivering them to the ICP-MS system for quantitative analysis.
In this article, we detail the main components of a GED, some of its configurations, its coupling to ICP-MS, the calibration approaches, and some selected results.
Gas Exchange Device
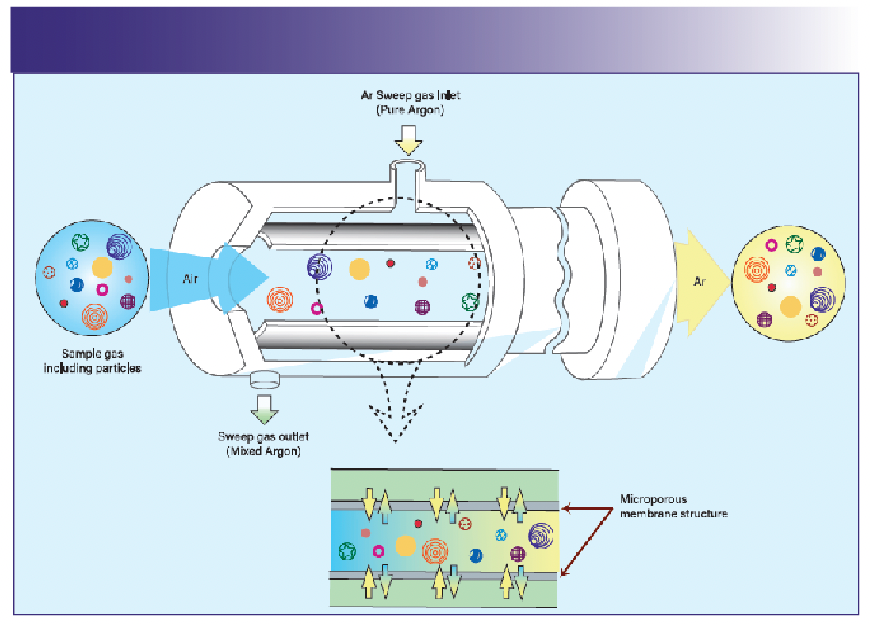
In simple terms, a GED is an introduction system that enables the direct introduction of gases for metal impurities analysis to an inductively coupled plasma coupled with a mass or optical spectrometer. This hardware consists of mass flow controllers, a pressurized exchange membrane, and few gas connections. The exchange membrane and the main gas connections are temperature-controlled. Figure 1 illustrates the operating principles of a GED and the sample gas flow through a microporous membrane residing in the center of a housing that is pressured by argon. The argon penetrates the exchange membrane, sweeping the sample gas. Meanwhile, the organometallic molecules and particles remain in the center channel. These contaminants are then delivered to the ICP-MS platform in a stream of argon that maintains plasma robustness.
FIGURE 1: Schematic diagram of the exchange membrane in a GED.
Configurations and Calibration Approaches
As mentioned earlier, the semiconductor and electronics industries utilize many different gases for various applications. In this section, we discuss various configurations and calibration approaches depending on the sample gas chemical and physical properties.
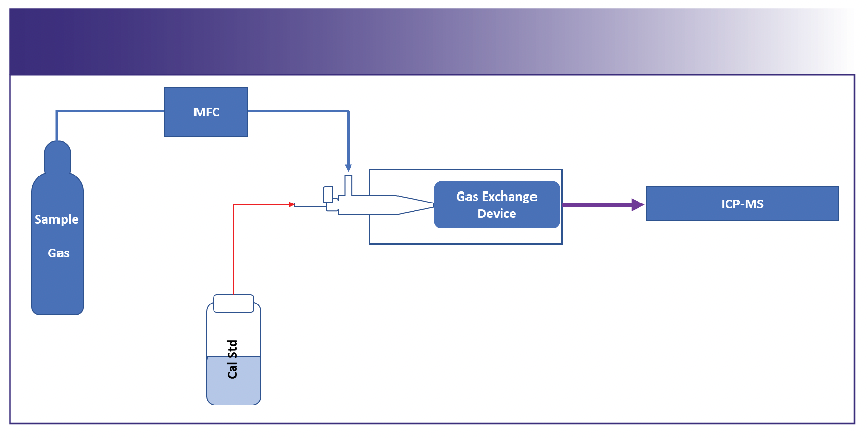
Inert nontoxic gases: The sample gas can be used in a similar manner, replacing argon as the nebulizer gas. The generated aerosol will enter the GED, and the sample gas gets exchanged while the impurities remain in the central channel and get introduced to the ICP-MS instrument. Calibration via standard addition is performed (Figure 2).
FIGURE 2: GED configuration for inert nontoxic gases.
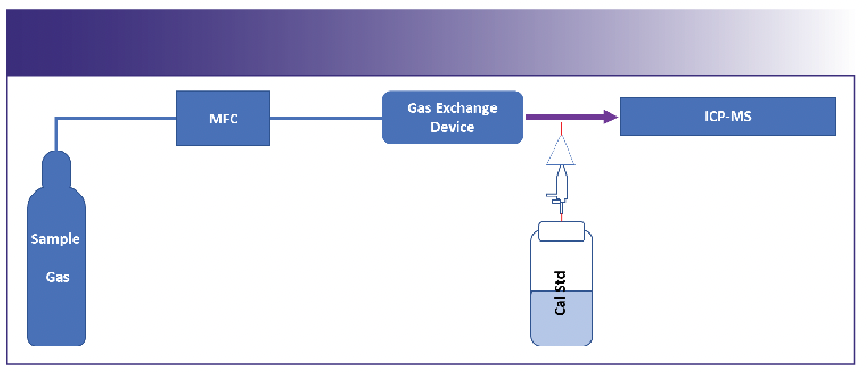
Toxic and moisture reactive gases: It is preferable to directly connect the sample gas to the GED while calibration is performed by method of standard addition (MSA) between the exit of the GED and the entrance to the ICP-MS system. These additions are performed with standard aerosol generation commonly used in the industry (Figure 3).
FIGURE 3: GED configuration for toxic gases.
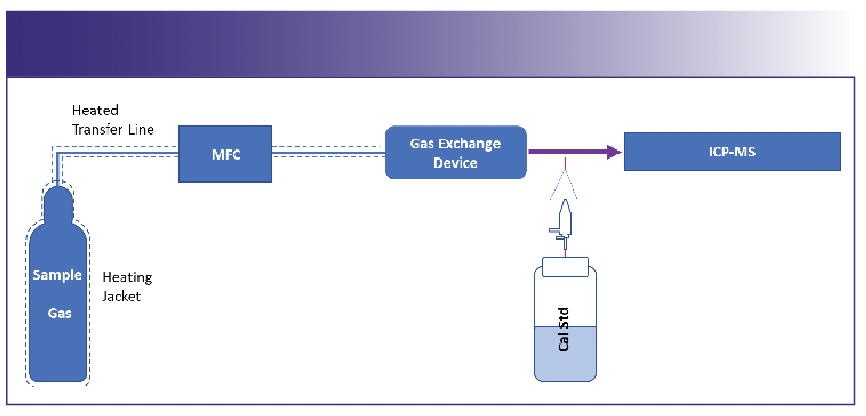
Low-boiling temperature gases: For these gases, depending on their toxicity, either of the above configurations could be used. Consideration should be taken to ensure suitable heaters are installed from the sample gas tank to the entrance of the GED. In some cases, the sample gas can be mixed with an inert gas, like argon or nitrogen, prior to its introduction to the GED (Figure 4).
FIGURE 4: GED configuration for low boiling temperature gases.
Integration with ICP-MS
The ICP-MS platform is a key component in the GED–ICP-MS system. It offers high-resolution mass spectrometry (HRMS) capabilities, enabling the precise quantification of metallic impurities even at trace levels (9). In all configurations, the output of the GED is connected to the ICP-MS injector via polytetrafluoroethylene (PTFE) or perfluoroalkoxy (PFA) tubes using commonly used industry-standard fittings.
Benefits of Combining GED with ICP-MS
Enhanced Sensitivity and Detection Limits: The sensitivity of ICP-MS complements the GED’s ability to deliver gas samples directly. This combination results in excellent detection limits and accuracy for metallic impurity analysis.
Removal of Gas Matrix: The GED removes the matrix or background associated with the gas that is being tested, helping with the normalization of the plasma and ensuring better calibration and more accurate results.
Improved Data Accuracy: Interference removal by dynamic reaction cells (DRC) technology enhances measurement accuracy. For accurate interference removal, the reaction should be controlled by utilizing cell features such as bandpass filtering for the low-mass cut off, the ion speed, and the reaction gas flow. Furthermore, multi-quadrupole technology can refine these reactions, ensuring that even complex matrices and low-level impurities are accurately quantified.
Streamlined Workflow: The integration of the GED with ICP-MS creates a seamless workflow from sample introduction to data analysis. The direct injection of gases into ICP-MS via the GED minimizes sample handling and preparation, reducing potential sources of error and contamination.
Single Particle Analysis Capability: For accurate particulate analysis, fast acquisition, short dwell times, and no settling times are required. To further improve the detection limit of nanoparticulate in the gasses, collisional focusing and Axial field technology (AFT) can be used to compress the ion plume of the particle before reaching the detector, allowing the user to monitor the gas for metallic nanoparticles.
Advanced Calibration and Data Analysis:ICP-MS technologies that support sophisticated calibration methods, including standard addition and internal standardization, are critical for maintaining data accuracy. Combined with the GED, this enables comprehensive and precise analysis of a wide range of metallic impurities.
Case Example: The Analysis of Carbon Monoxide and Hydrochloric Gas
In a collaborative project with International Analytical Solutions (IAS Inc.), the NexION 5000 (PerkinElmer U.S LLC) ICP-MS system was successfully integrated with a GED for analyzing hydrochloric acid (HCl) gas. This integration also included a metal standard aerosol generation (MSAG) system to automatically and accurately generate a calibration. The MSAG-GED–ICP-MS system opens up new opportunities for analyzing metallic impurities in gases, offering a powerful analytical solution that meets the demanding needs of the semiconductor industry. Particle measurement by GED using fast acquisition and short dwell times allowed the detection of a few nanometers (nm) of particles in the HCl gas. This application demonstrated the effectiveness of the system in delivering high-resolution data with improved detection limits and robustness compared to traditional methods.
Results
By partnering with Bio & Analytical Solution Supplier (BASS) and IAS Inc. to integrate the NexION ICP-MS platform with their respective GEDs, applications were developed for the analysis of metallic impurities in carbon monoxide (CO) and hydrochloric (HCl) gas, respectively.
This section discusses selected data from both applications, showcasing the performance of GED–ICP-MS versus impinger, focusing on the detection limits, robustness, repeatability, and ease of use.
Detection Capability
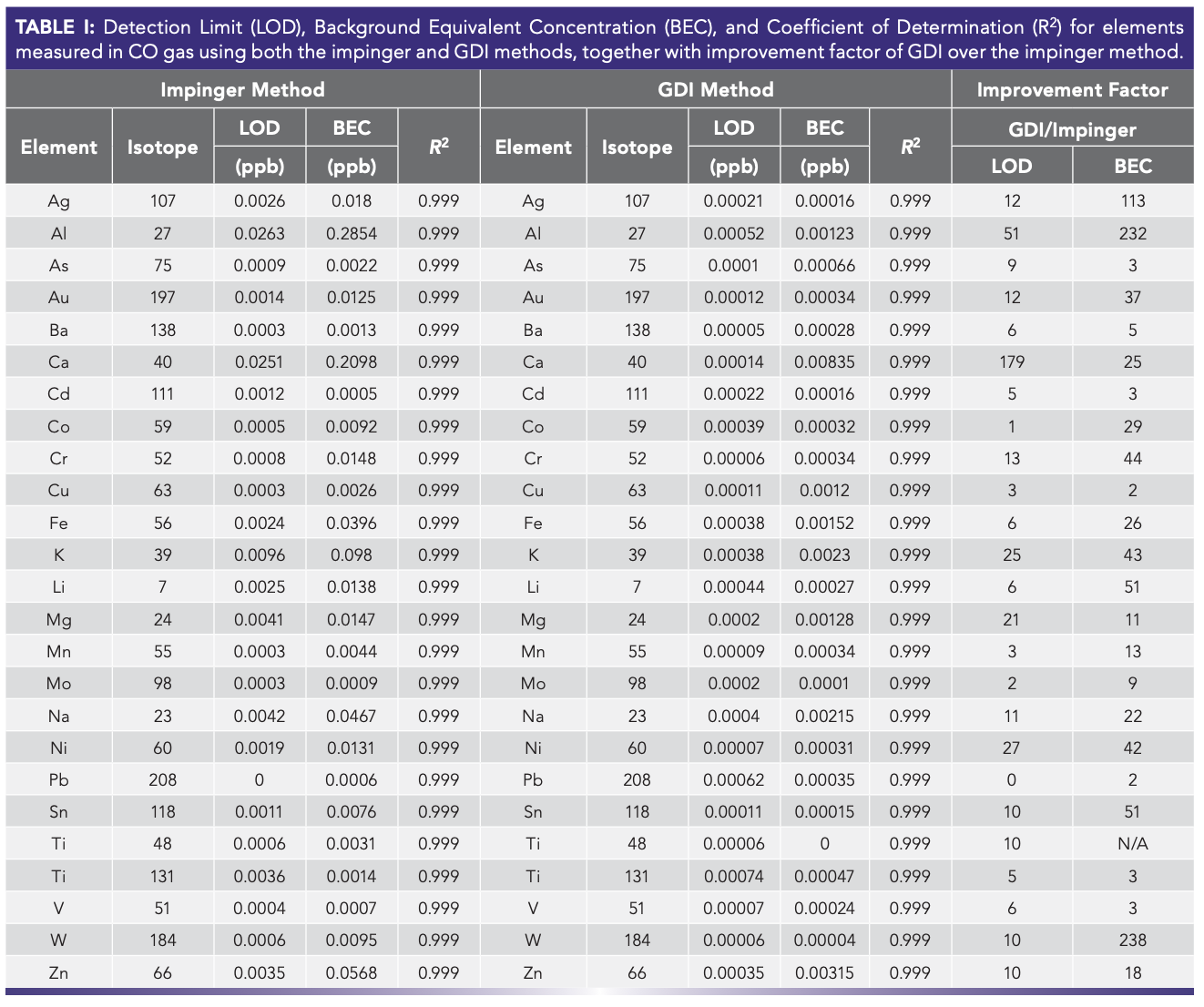
As mentioned earlier, gas purity is the most important parameter this industry demands. GED–ICP-MS outperformed the impinger in both limit of detection (LOD) and background equivalent concentration (BEC) by factors ranging from 10 to more than 100-fold (Table I). This noticeable improvement in BEC and LOD derives from the direct injection ability of the GED, surpassing the need for a collection media used in the impinger. These collection media are made from ultrapure water and other reagent chemicals that contribute to the background.
Robustness and Repeatability
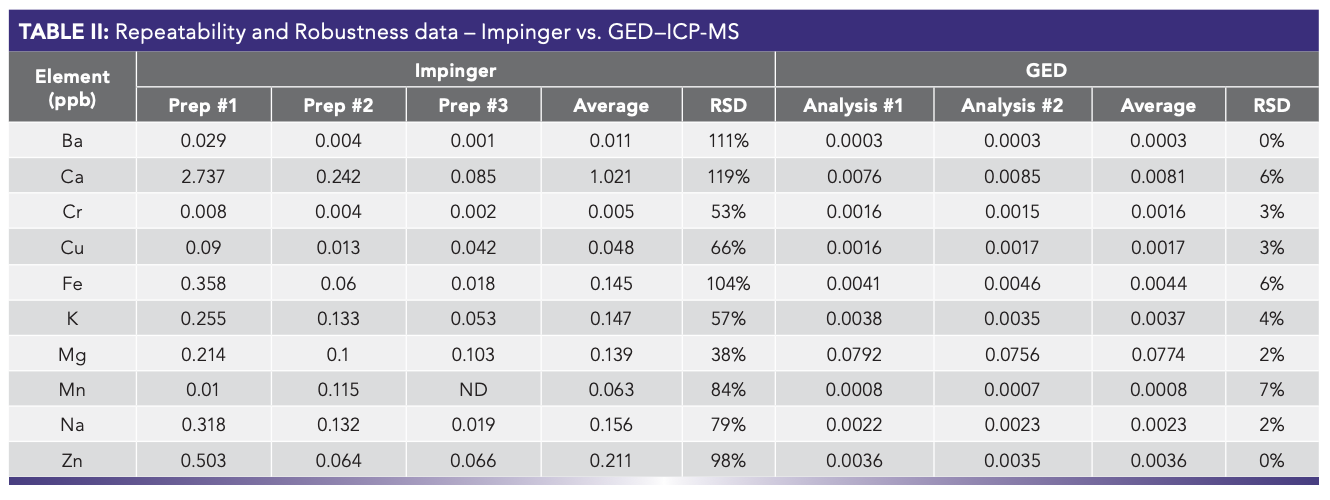
Robustness and repeatability were evaluated, comparing three different sample preparation methods using the impinger. The same sample gas was also analyzed by GED–ICP-MS two times, three replicates per analysis. Observing the relative standard deviation (RSD) for the selected elements (Table II), it can be seen that the high values for the common contaminants like Ca, Fe, Na, and Mn indicate that contamination usually results from the various sample preparation steps or cross-contamination from previous samples. The GED results show excellent repeatability and robustness. When comparing the obtained metal concentration between the impinger and the GED, it can be clearly seen that the latter reported lower concentrations as the impinger concentration will have contribution from ultrapure water, the reagent, and possibly random cross-contamination. The direct introduction and analysis of a sample gas by GED–ICP-MS eliminates those factors.
Productivity
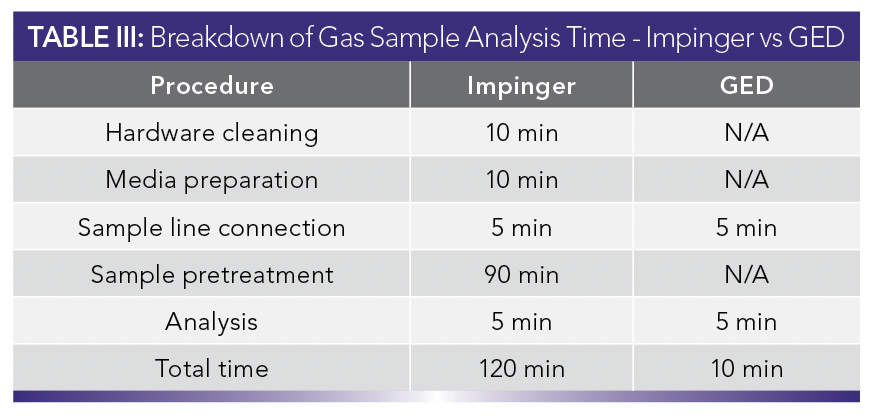
Productivity is measured by the time required to complete an analysis. This encompasses getting the instrument ready, preparing the sample, and analyzing it. Table III highlights the time required for each step needed to achieve a gas sample analysis. The impinger method is 12 times slower than the GED, with the bulk of the time being spent on sample pretreatment.
The advantages of gas direct injection analysis by GED–ICP-MS compared to the impinger is demonstrated through its ease of use and ability to analyze gas samples without prior treatments. This technology enables many use-case applications, such as performing quality control on gas cylinders, gas manifolds, and monitoring degradation of the extensive network of gases used in semiconductor fabrication plants.
Future Advancements in GED Technology
Looking ahead, developments in GED technology could include improvements in membrane materials for better gas exchange efficiency or enhanced integration with other analytical techniques. Innovations might also focus on expanding the range of detectable impurities and reducing operational costs. As the semiconductor industry continues to evolve, these advancements will play a crucial role in meeting emerging challenges.
Further Automation
The GED and ICP-MS combination can be further enhanced by automating the calibration and sampling of the semiconductor-grade gases, which ensures that laboratories needing to carry out these measurements will have the sophistication, flexibility, throughput, and accuracy needed to monitor all gas types.
Conclusion
GED–ICP-MS represents a significant advancement in the analysis of metallic impurities in specialty gases. Its ability to provide real-time, accurate results without the need for extensive sample preparation sets it apart from traditional methods. This technology not only improves detection limits and repeatability, but it also enhances productivity by reducing analysis time. As industries continue to push the boundaries of technology, the GED–ICP-MS system is poised to play a pivotal role in maintaining high standards of gas purity and performance.
References
(1) Jones, A. C.; Hitchman, M. L. Chemical Vapour Deposition: Precursors, Processes and Applications; Royal Society of Chemistry, Cambridge, UK 2009. DOI: 10.1039/9781847558794
(2) Geiger, W. M.; Raynor, M. W. Trace Analysis of Specialty and Electronic Gases; New Jersey, John Wiley & Sons, 2013. DOI: 10.1002/9781118642771
(3) Stephan, C.; Ryu, C. System for Introducing Particle-Containing Samples to an Analytical Instrument and Methods of Use, US Patent 11581177B2 US CA KR WO EP TW, 2019. https://patents.google.com/patent/US11581177B2/en (accessed 2024-9-11)
(4) Park, W. H.; Park, J. G., Analysis of Metallic Impurities in Carbon Monoxide Gas by Gas Direct Injection - ICP-MS. PerkinElmer 2024. https://content.perkinelmer.com/library/app-analysis-of-metallic-impurities-in-carbon-monoxide-gas-by-gas-direct-injection-icp-ms.html (accessed 2024-9-11).
(5) Suzuki, K.; Nishiguchi, K.; Kawabata, K. Direct Analysis of Metallic Impurities in Hydrochloric Gas Using Gas Exchange Device (GED) ICP-MS. PerkinElmer 2022. https://www.semanticscholar.org/paper/Direct-Analysis-of-Metallic-Impurities-in-Gas-Using-Suzuki-Nishiguchi/e3a56fe0db5f5ba4de4ff16a3bedb9b51fd0fb56, (accessed 2024-9-11)
(6) Deloitte’s Center for Technology, Media & Telecommunications (TMT Center). 2024 Global Semiconductor Industry Outlook. Deloitte 2024. https://www2.deloitte.com/content/dam/Deloitte/us/Documents/technology-media-telecommunications/us-tmt-semiconductor-industry-outlook-2024.pdf?trk=public_post_comment-text (accessed 2024-9-11).
(7) SK Hynix Newsroom. Semiconductor Front-End Process Episode 4: Etching Fine and Identical Wafer Patterns. SK Hynix Inc 2023. https://news.skhynix.com/semiconductor-front-end-process-episode-4(accessed 2024-9-11)
(8) PerkinElmer. An Interactive Guide to Semiconductor & Electronics Contaminant Testing Solutions. PerkinElmer 2024. https://www.perkinelmer.com/libraries/bro_64052_solutions-for-semiconductors-and-electronics (accessed 2024-9-11)
(9) PerkinElmer. Inductively Coupled Plasma Mass Spectrometry (ICP-MS). PerkinElmer 2024. https://www.perkinelmer.com/category/inductively-coupled-plasma-mass-spectrometry-icp-ms (accessed 2024-9-11).
About the Authors
Chady Stephan, Aaron Hineman, and Ruth Merrifield are with PerkinElmer. ●


Atomic Perspectives: Highlights from Recent Columns
March 3rd 2025“Atomic Perspectives,” provides tutorials and updates on new analytical atomic spectroscopy techniques in a broad range of applications, including environmental analysis, food and beverage analysis, and space exploration, to name a few. Here, we present a compilation of some of the most popular columns.
Pittcon 2025: Highlighting Talks on Atomic Spectroscopy
February 26th 2025At Pittcon this year, there will be numerous sessions dedicated to spotlighting the latest research that uses atomic spectroscopy or elemental analysis techniques. We highlight some of these talks below that might pique the interest of spectroscopists and researchers attending the conference this year.