Characterization of Street Drugs Using Handheld Fourier Transform Raman Spectroscopy
Handheld Raman spectroscopy offers a rapid and mobile technique for determining drugs nondestructively. The technique offers further advantage for analyzing drug products (DPs) thanks to its ability to measure samples through packaging, thus preserving evidence integrity and continuity. This research evaluated the use of handheld Fourier transform (FT)-Raman spectroscopy for characterization of street DPs (n = 254) of diverse formulations. Raman spectra of DPs inside their packaging were collected and matched against the instrumental in-built library, and then exported to Matlab 2020a for offline spectral interpretation. Reference analysis was confirmed using gas chromatography–mass spectrometry (GC–MS) and infrared (IR) spectroscopy. The main drugs present were 1,3-trifluoro- methylphenylpiperazine, cocaine, ketamine, 3,4-methylenedioxymethamfetamine, mephedrone, and 1-benzylpiprerazine. The main impurities present in DPs were benzocaine, caffeine, and lactose. The impurities affected the Raman signature (enhanced or inhibited) of DPs, enabling profiling of DPs and visualizing patterns among different products. These latter patterns were featured in the similarities highlighted by the correlation coefficient values between different DPs, and by adjacent clusters of different DPs envisaged in the principal component analysis scores plot.
Crime and violent behaviors have increased because of the widespread use of illicit drugs in contemporary societies. Amsterdam (1027.7), London (701.1), and Copenhagen (780 mg/1000 p/day) had the highest weekend daily mean cocaine usage in 2020 (1). Drug addiction endangers public health, safety, and welfare, as well as the security of the nation. Researchers and authorities require quick and accurate drug detection methods that can be adapted to the ever-increasing spectrum of illicit drugs. Currently, the primary goal of drug analysis is to detect and identify drugs of abuse.
Analytical techniques such as thin layer chromatography, gas chromatography–mass spectrometry (GC–MS), gas chromatography-flame ionization detection (GC-FID), high performance liquid chromatography (HPLC), and liquid chromatography–mass spectrometry (LC– MS) have all been proposed, developed, and used to detect illicit drugs (2). These techniques provide reliable and accurate results in drug analysis, but they are costly, time consuming, and destructive, which limits their practical ability. Vibrational spectroscopic methods, such as Fourier transform infrared (FT-IR) and Raman, address the aforementioned limitations, and they have also been applied for qualitative and quantitative investigations of illicit drugs (3,4).
Raman spectroscopy measures the vibrational modes of a sample. The spectrum is a wavelength distribution of bands that correspond to molecular vibrations in the sample under investigation. The Raman band frequencies and intensities could be used to identify and quantify the measured samples (5). Raman spectroscopy generates molecular-specific spectra with minimum or no sample preparation, permitting non-destructive in situ analysis for different forms of drugs (liquids, tablets, and powders) (6), which is very useful when a substantial number of samples need to be analyzed, and it is essential for avoiding sample contamination, reducing analysis time, and protecting the evidence under investigation. Raman spectroscopy has been used in a variety of applications, including forensics (6), industrial process control (7), biomedical purposes (8), and medical diagnostics (9). When identifying drugs, Raman can distinguish between salts, stereoisomers, and homologs with a sample size of more than 5 mg (10), and it also can reveal a high level of specificity for drugs concealed in alcohol (11). Minor variations in chemical structures can be identified, thus providing a characteristic spectrum for each chemical compound and making the identification of drugs more reliable (12). Raman spectroscopy has recently been used to check drug contents within their packaging (13), evaluate the composition and homogeneity of drug tablets (14), classify street drugs (15), and determine drug authenticity (16). Raman spectra of drug blends are often highly complex because they include spectral features corresponding to all components in the sample. Consequently, chemometric methods must be used to obtain quantitative information from complex spectra (17). This study complements previous studies in evaluating the use of handheld Raman spectroscopy for on-spot identification and characterization of street drugs in their packaging.
Experimental
Street samples of drug products (DPs) (n = 254) were measured as received, and included powders, tablets, capsules, liquids, and rocks of different colors. Samples were measured through glass vials using a handheld FT-Raman spectrometer equipped with 490 mW of laser power, a 1064 nm laser wavelength, and cooled InGaAs detector. Spectra were collected over the wavenumber range of 250–2000 cm-1 with a spectral resolution of 10 cm-1. The in-built spectral algorithm was used to search matches of the drug samples against the instrumental libraries. For each sample, the library algorithm gave matches for the drugs or additives that showed spectral features. For offline analysis, the spectra of the products were interpreted considering spectral quality, functional groups, and similarities among products using correlation in wavenumber space (CWS) and principal component analysis (PCA) algorithms. Spectral quality was evaluated for the measured drug products, considering the low spectral resolution in FT-Raman (18). For the CWS method, the momentum product was calculated between the spectra of different products reporting a common drug. A threshold of 0.95 or more was considered a match and vice versa (19). For PCA, drug products’ spectra were explored for patterns among their scores. A false negative was seen when a drug product score was not grouped with product scores of the same drug, whereas false positives were seen when a drug product score was clustered with scores of different drugs. Reference analysis was conducted using GC–MS and attenuated total reflectance-Fourier transform infrared (ATR-FT-IR) spectroscopy. GC–MS and ATR-FT-IR confirmed the identity of the drugs or common impurities present in DPs, respectively (Table I).
Results and Discussion
Out of the measured DPs, 153 contained drugs or drug mixtures, most of which were not pure. The main drugs found were 1,3-trifluoromethylphenylpiperazine (TFMPP) (n = 42), cocaine (n = 36), ketamine (n = 29), 3,4-methylenedioxymethamfetamine (MDMA) (n = 24), mephedrone (n = 9), 1-benzylpiprerazine (BZP) (n = 7) butylone (n = 2), 3,4-methylenedioxypyrovalerone (MDPV) (n = 2), 1-(3-chlorophenyl)piperazine (mcPP) (n = 2), dibenzylpiperazine (DBZP) (n = 1), and 1-methyl-4-benzylpiperazine (mBZP) (n = 1).
The main impurities detected were caffeine (n = 57) and benzocaine (n = 35), which were both Raman active. Lidocaine and procaine were detected less than benzocaine, and they were found in seven and two products, respectively. Benzocaine and lidocaine were mainly found as impurities in cocaine drug products, alongside other impurities such as phenacetin and caffeine. Thus, the pattern of adulterants varied between DPs. For instance, caffeine was also an adulterant in MDMA and piperazine derivatives. On the other hand, lactose was a bulking agent reported in diazepam tablets, and it contributed to the low Raman activity of these products. Likewise, dextrose and sucrose were also found in DPs of tablet- and powder-formulations and contributed to the low Raman activity of these products. Hence, drugs adulterated with the aforementioned sugars were not detected in tablets or powders using Raman spectroscopy where the Raman signal of these tablets was masked by lactose fluorescence.
Spectral quality of DP
Spectral quality was evaluated considering these parameters: the number of peaks (N); maximum peak position and intensity; wavenumber range; and signal-to-noise ratio (S/N) (19). In this respect, synthetic piperazine, cocaine, and mephedrone products showed strong Raman activity, ketamine products showed medium Raman activity, and MDMA products showed low Raman activity.
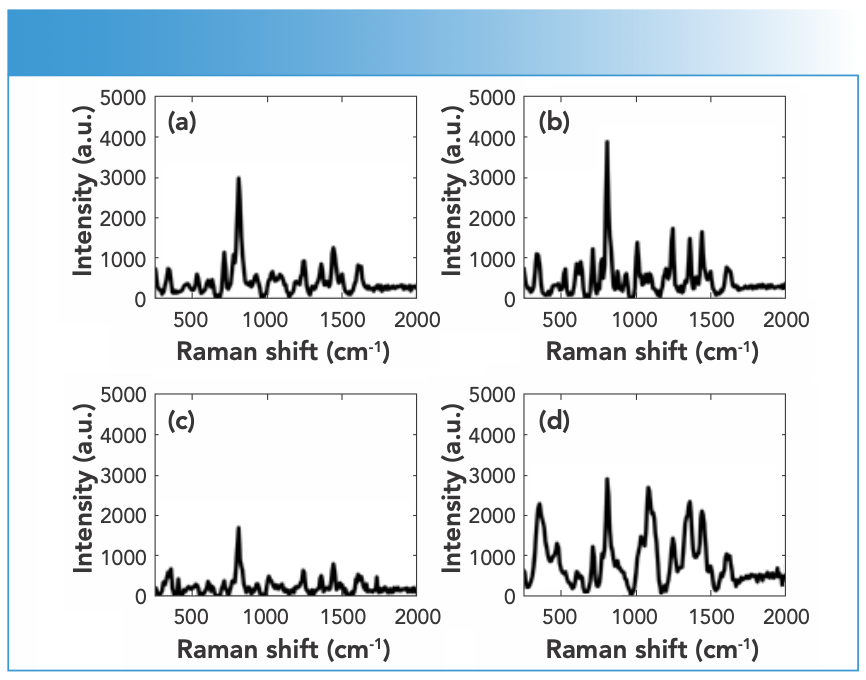
Synthetic piperazines’ spectral quality varied depending on the purity of the DPs and the types of impurities present in them (Figure 1). TFMPP samples showed stronger Raman activity than other piperazines, and that was related to their being either pure or cut with caffeine that in turns was Raman active. The ranges of N, maximum intensity, and S/N values for piperazine derivatives were 15–25, 1500–15000 a.u., and 10–20, respectively. Cocaine products demonstrated strong Raman activity, but that depended on the adulterants present in the cocaine sample, which affected the Raman activity (Figure 2). For cocaine, Raman active samples, N, maximum intensity, and S/N were in the range of 11–27, 1,744–51,242 a.u. and 15–39, respectively. Similarly, mephedrone showed strong Raman activity with ranges of N, maximum intensity and S/N in the ranges of 25–29, 1500–8000 a.u., and 18–37, respectively (Figure 3). Ketamine products showed medium Raman activity with N, maximum intensity, and S/N in the ranges of 16–30, 700–4000 a.u., and 5.75–39, respectively (Figure 4). On the other hand, MDMA products exhibited low Raman activity with low S/N < 20), despite the high N in most spectra (up to 25) (Figure 5). The maximum intensity seen for MDMA products was in the range of 1500–5000 a.u.
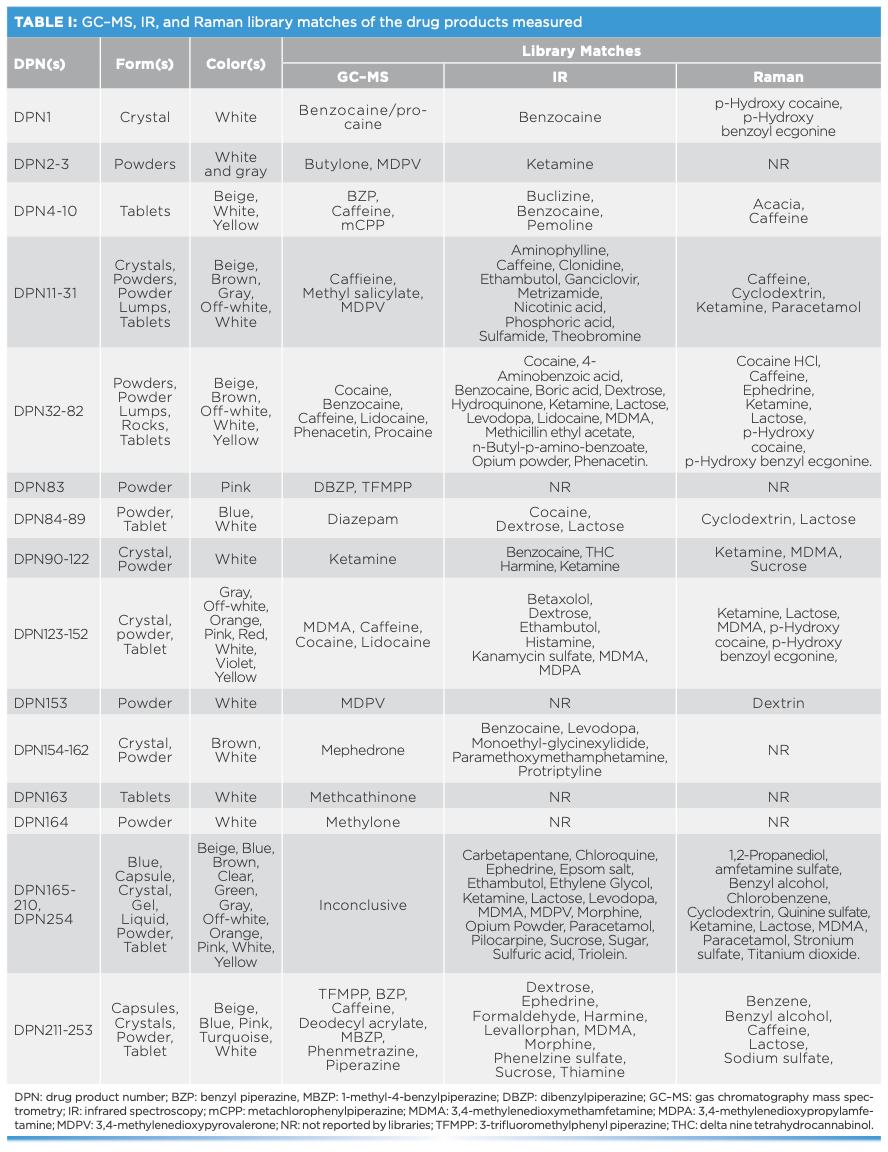
FIGURE 1: Raman spectra of (a) TFMPP and (b) BZP DPs measured using an FT-Raman spectrometer equipped with 1064-nm laser excitation wavelength.
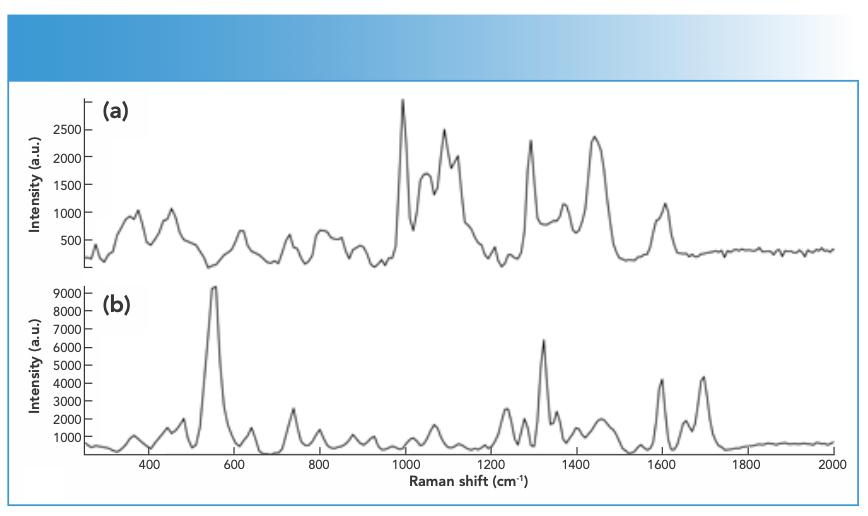
FIGURE 2: Raman spectra of DPs containing (a) cocaine, (b) cocaine and benzocaine, and (c) cocaine and caffeine measured using an FT-Raman spectrometer equipped with 1064-nm laser excitation wavelength.
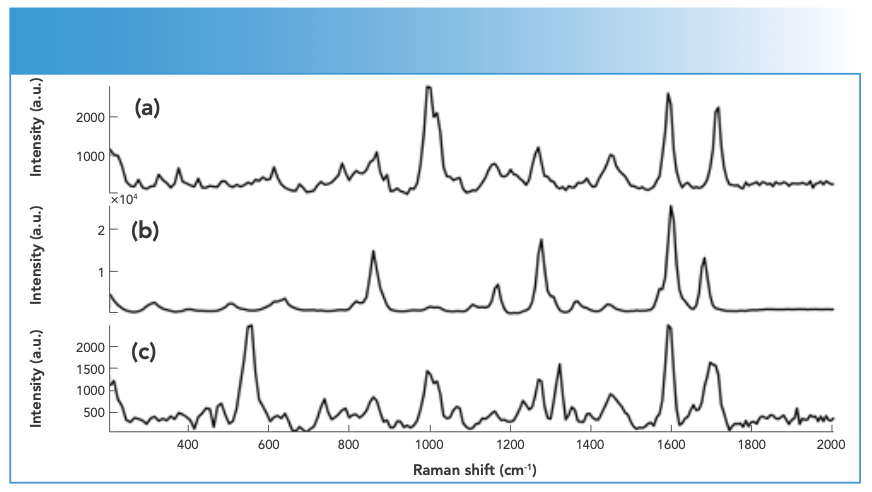
FIGURE 3: Raman spectra of mephedrone samples in crystal (black) and powder (red) forms measured using an FT-Raman spectrometer equipped with 1064-nm laser excitation wavelength.
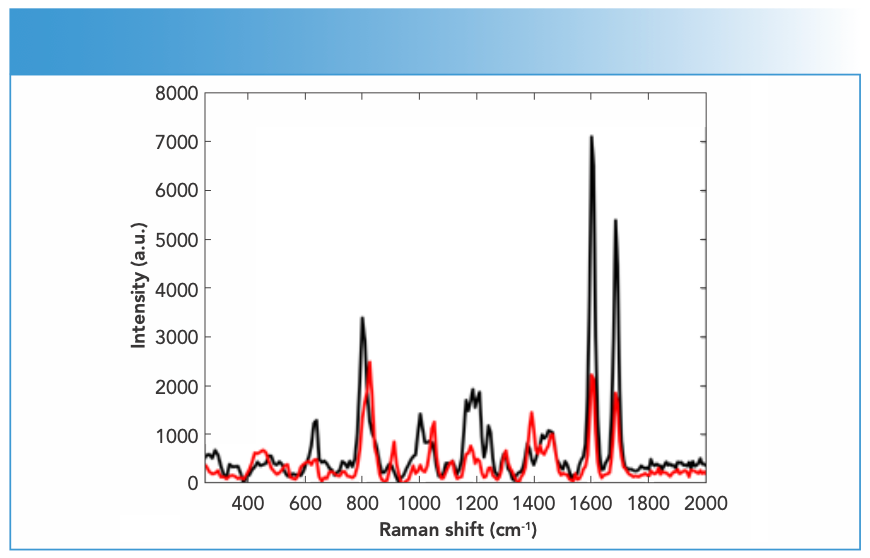
FIGURE 4: Raman spectra of ketamine DPs measured using an FT-Raman spectrometer equipped with 1064-nm laser excitation wavelength.
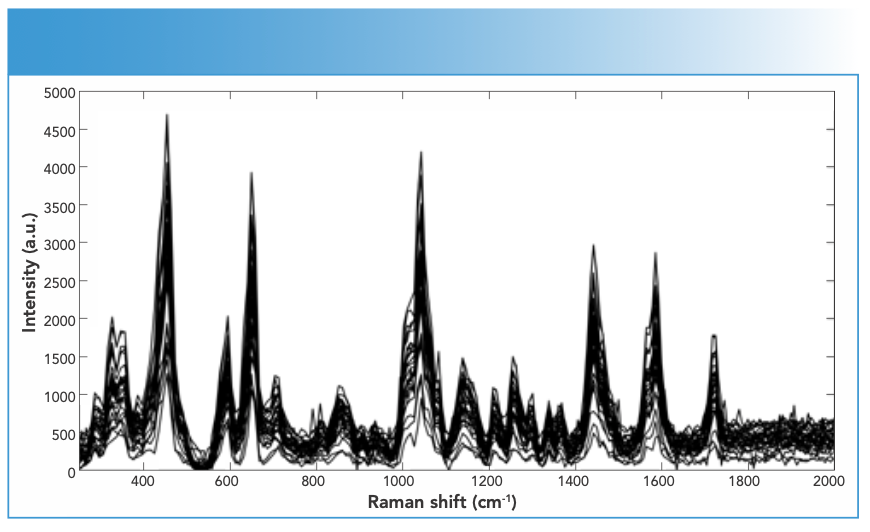
FIGURE 5: Raman spectra of MDMA DPs in the following colors or forms: (a) gray crystal, (b) white crystal, (c) orange powder, and (d) pink tablet measured using an FT-Raman spectrometer equipped with 1064-nm laser excitation wavelength.
Spectral Interpretation
Spectral interpretation showed characteristic scattering bands for the major drugs and impurities present in the measured DPs (Table II). Most drugs showed key bands between 500–1700 cm-1 (21–25). In this respect, Raman spectra indicated key differences between different drug analogues as well as base or salt forms of the same drug. For drug analogues, piperazine derivatives (TFMPP and BZP) could be differentiated over the ranges 500–1000 and 1300–1700 cm-1, respectively (Figure 1). Similarly, cocaine could be discriminated from cocaine HCl mainly in the range between 850–890 cm-1, where cocaine base showed three bands around 850, 870, and 890 cm-1 corresponding to the C-C stretch of the tropane ring in the cocaine base (20). On the other hand, cocaine HCl only showed two C-C stretching bands corresponding to the tropane ring around 850 and 890 cm-1 (20,21). Where benzocaine was present in cocaine DPs, it could be traced by the strong absorption intensity and by the characteristic bands in the range of 850–1700 cm-1 (Figure 2).
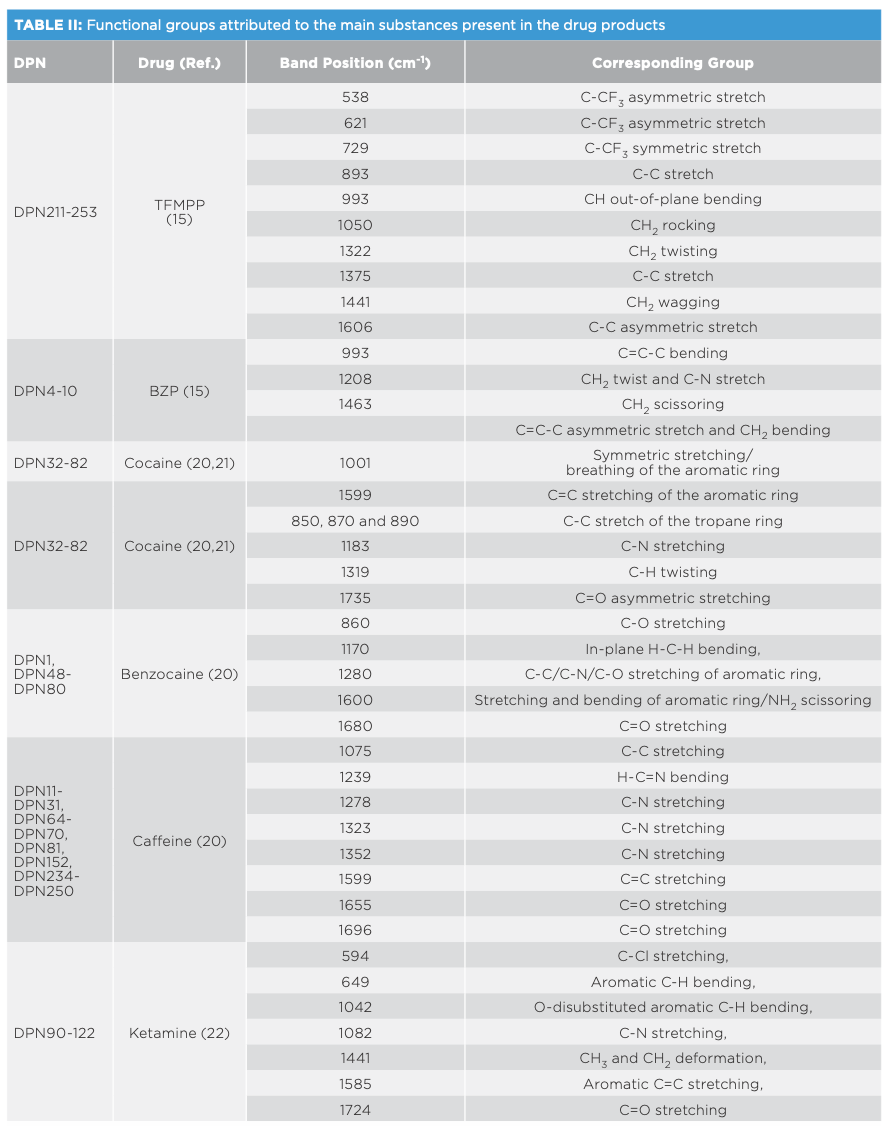
Classification of DPs
When CWS was applied to the spectra of the top encountered DPs, variable correlation (r value) ranges were observed depending on the amount of drug in DPs and on the purity of the specific DP (Figure 6). For instance, TFMPP-related products (DPN213–DPN254) did not show high r values against each other, and that they could not be considered a false negative because of the inconsistency in constituents in TFMPP products, indicating the presence of other additives, such as caffeine, dextrose, ephedrine, lactose, phenmetrazine, and sucrose. In this respect, the Raman activity of the constituents impacted the Raman scattering in terms of number of bands and Raman activity of the DP. For instance, caffeine had strong Raman scattering, and that influenced the signature of the TFMPP products containing them (DPN234–249). Nevertheless, the latter products contained impurities such as lactose that was only detected by Raman (and not GC–MS), which in turn influenced their Raman activity. Lactose and other sugars introduced fluorescence in the Raman spectra of TFMPP products (Figure 1). It is important to mention that the physical properties of the DPs did not influence the Raman signature. Hence, TFMPP products of similar chemical makeup and different physical characteristics (formulation, color) showed high r values against each other. For example, DPN217 (white tablet formulation) showed an r value of 0.95 against DPN220 (white crystal formulation). Also, DPN228 (pink tablets), DPN235 (white tablets), and DPN246 (blue tablets) showed r values of 0.95 against each other. It is worth noting that no false positives were seen for TFMPP-DPs except against a DP containing BZP, and this mismatch could be related to the structural similarity between TFMPP and BZP.
FIGURE 6: (a) Correlation map of the Raman spectra of TFMPP (1:42), cocaine (43:78), ketamine (79:107), MDMA (108:131), caffeine (132:150), mephedrone (151:159), and BZP (160:166) DPs, where r values above 0.95 are marked in yellow and those below 0.95 are marked in red. (b) PCA scores plot of the Raman spectra of TFMPP (black circle), cocaine (blue square), ketamine (red star), MDMA (green triangle), mephedrone (cyan triangle) and BZP (black star) DPs.
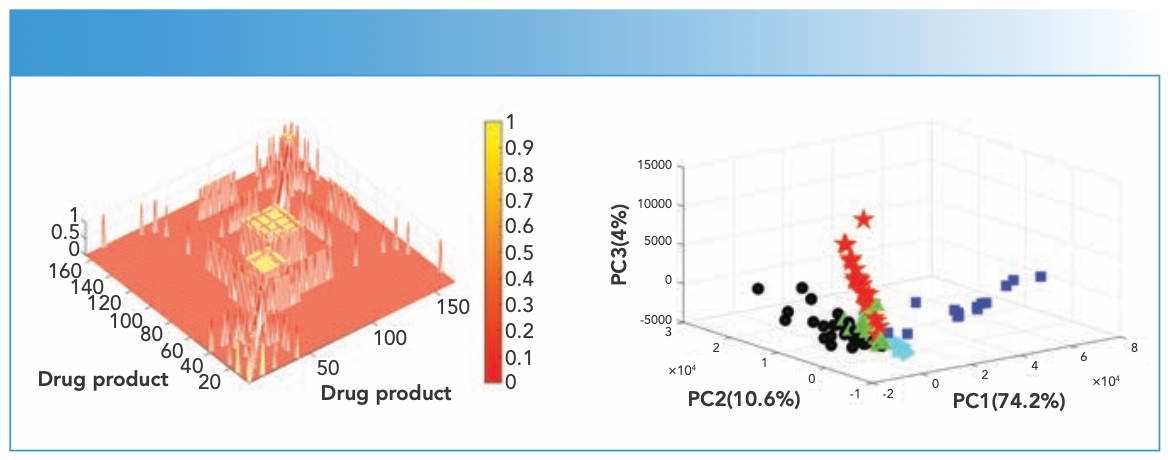
Cocaine DPs featured less false negatives, and that could be attributed to the consistency in the types of impurities detected in the measured cocaine DPs. For instance, samples with a cocaine/benzocaine mixture (DPN48–DPN62) showed r values > 0.95 against each other where both constituents in the matrix were Raman active and were strong scatterers. Where more than two constituents were present in a cocaine DP, the r value changed, and that also depended on the Raman activity of the constituents. Overall, the constituents were unique to cocaine mixtures where no false positives were observed with cocaine mixtures against other drugs. Similar patterns in r values results were seen for ketamine samples that were mainly pure, and showed r values > 0.95 against each other and r values < 0.95 against different drugs. Only four ketamine DPs showed low r values against other ketamine products (one Raman active and three Raman inactive samples). The remaining DPs (containing MDMA and mephedrone) showed false positives and false negatives related to the high number of impurities present in them, and that had been reported in the literature, especially in mephedrone DPs.
Hence, CWS results were only conclusive when the exact number of impurities were present in the reference and test tablets. Considering the aforementioned challenge in identification, PCA recognized patterns in the DPs scores that corresponded to spectral features contributing to high variances (Figure 6). The first three PCs contributed 88.8% of the variance among the DPs’ spectra, with cocaine DPs’ scores recording the highest variance. This was followed by ketamine DPs’ scores and TFMPP DPs’ scores. Cocaine, ketamine, and TFMPP DPs’ scores showed three distinct clusters with slight overlap in few scores, and that could be related to the caffeine or lactose that were impurities present in DPs of the three drugs. Some MDMA DPs that contained lactose overlapped with products of the same impurity. On the other hand, mephedrone DPs were clearly distinguished from other DPs, and that showed their unique composition.
Conclusion
In summary, handheld FT-Raman spectroscopy complemented GC–MS and IR in characterizing street DPs. It indicated information regarding certain impurities that could not be detected by GC–MS (such as lactose and sucrose). Another advantage encountered over IR was the ability of Raman to characterize Raman active drugs nondestructively regardless of the physical differences between the matrices (tablets, powders, or liquids). However, some limitations were encountered in processing the spectra of the drug products relating to the sensitivity of Raman in detecting drugs that contained impurities, especially fluorescent ones such as lactose. Moreover, the presence of multiple impurities introduced a challenge in the identification and characterization of the drug product. Further understanding of Raman spectra, particularly in samples of multiple impurities, is needed. Therefore, future work will consider investigating drugs in mixtures using a more quantitative approach.
Acknowledgments
The authors would like to thank Bournemouth University for access to drug products and instrumentation. Special thanks for Amy Williams, Chelsea Bailey, and Angela Buffardi for their help with spectral data collection.
References
(1) European Drug Report: Trends and Developments (European Monitoring Centre for Drugs and Drug Addiction, Libson, Portugal, September 2020).
(2) United Nations Office on Drugs and Crime (UNODC), Recommended Methods for the Identification and Analysis of Amphetamine, Methamphetamine and Their Ring-Substituted Analogues in Seized Materials (United Nations, New York, NY, 2006).
(3) Izake, E.L. Forensic and Homeland Security Applications of Modern Portable Raman Spectroscopy. Forensic Sci. Int. 2010, 202 (1–3), 1–8. DOI: 10.1016/j.forsciint.2010.03.020
(4) Praisler, M.; Van Bocxlaer, J.; De Leenheer, A.; Massart, D.L. Chemometric Detection of Thermally Degraded Samples in the Analysis of Drugs of Abuse with Gas Chromatography-Fourier Transform Infrared Spectroscopy. J. Chromatogr A 2002, 962, 161–173. DOI: 10.1016/s0021-9673(02)00536-8
(5) Yang Y.; Thomas, R. The Benefits of a High-Performance, Handheld Raman Spectrometer for the Rapid Identification of Pharmaceutical Raw Materials. Am. Pharm. Rev. 2012, 15, 1–7.
(6) Smith E.; Dent, G. Modern Raman Spectroscopy: A Practical Approach (John Wiley & Sons, Chichester, United Kingdom, 2005).
(7) Gilbert, A.S.; Lancaster, R.W. “IR and Raman Spectroscopy. Industrial Applications,” in Encyclopedia of Spectroscopy and Spectrometry, J.C. Lindon, G.E. Tranter, and D.W. Koppenaal, eds. (Academic Press, Oxford, United Kingdom, 3rd ed., 2017), pp. 394–407.
(8) Krafft C.; Popp, J. The Many Facets of Raman Spectroscopy for Biomedical Analysis, Anal. Bioanal. Chem. 2015, 407 (3), 699–717. DOI: 10.1007/s00216-014-8311-9
(9) Kong, K.; Kendall, C.; Stone, N.; Notingher, I. Raman Spectroscopy for Medical Diagnostics — From In-Vitro Biofluid Assays to In-Vivo Cancer Detection. Adv. Drug Deliv. Rev. 2015, 89, 121–134. DOI: 10.1016/j.addr.2015.03.009
(10) Lanzarotta, A.; Witowski, M.; Batson, J. Identification of Opioids and Related Substances using Handheld Raman Spectrometers. J. Forensic Sci. 2020, 65 (2), 421–427. DOI: 10.1111/1556-4029.14217
(11) Burnett, A.D.; Edwards, H.G.M.; Hargreaves, M.D.; Munshi, T.; Page, K. A Forensic Case Study: The Detection of Contraband Drugs in Carrier Solutions by Raman Spectroscopy, Drug Test. Anal. 2011, 3 (9), 539–543. DOI: 10.1002/dta.169
(12) Mazurek S.; Szostak, R. Quantitative Determination of Diclofenac Sodium and Aminophylline in Injection Solutions by FT-Raman Spectroscopy. J. Pharm. Biomed. Anal. 2006, 40 (5), 1235–1242. DOI: 10.1016/j.jpba.2005.09.019
(13) McCreery, R.L.; Horn, A.J.; Spencer, J.; Jefferson, E. Noninvasive Identification of Materials Inside USP Vials with Raman Spectroscopy and a Raman Spectral Library. J. Pharm. Sci. 1998, 87 (1), 1–8 (1998). DOI: 10.1021/js970330q
(14) Matousek, P.; Parker, A.W. Bulk Raman Analysis of Pharmaceutical Tablets. Appl. Spectrosc. 2006, 60 (12), 1353–1357. DOI: 10.1366/000370206779321463
(15) West, M.J.; Went, M.J. Detection of Drugs of Abuse by Raman Spectroscopy. Drug Test Anal. 2011, 3 (9), 532–538. DOI: 10.1002/dta.217
(16) Kalyanaraman, R.: Dobler, G.; Ribick, M. Portable Spectrometers for Pharmaceutical Counterfeit Detection. Am. Pharm. Rev. 2010, 13 (3), 38–45.
(17) Müller, J.; Knop, K.; Wirges, M.; Kleinebudde, P. Validation of Raman Spectroscopic Procedures in Agreement with ICH Guideline Q2 with Considering the Transfer to Real Time Monitoring of an Active Coating Process. J. Pharm. Biomed. 2010, 53 (4), 884–894. DOI: 10.1016/j.jpba.2010.06.016
(18) Vítek, P.; Ali, E.M.; Edwards, H.G.; Jehlička, J.; Cox, R.; Page, K. Evaluation of Portable Raman Spectrometer with 1064 nm Excitation for Geological and Forensic Applications. Spectrochim. Acta A 2012, 86, 320–327. DOI: 10.1016/j.saa.2011.10.
(19) Assi, S. Raw Material Identification Using Dual Laser Handheld Raman Spectroscopy. Eur. Pharm. Rev. 2013, 18, 25-31.
(20) Penido, C.A.F.d.O.; Silveira Jr, L.; Pacheco, M.T.T. Quantification of Binary Mixtures of Cocaine and Adulterants Using Dispersive Raman and FT-IR Spectroscopy and Principal Component Regression. Instrum. Sci. Technol. 2012, 40 (5), 441–456.
(21) Carter, J.C.; Brewer, W.E.; Angel, S.M. Raman Spectroscopy for the in Situ Identification of Cocaine and Selected Adulterants. Appl. Spec. 2000, 54 (12), 1876–1881. DOI: 10.1366/0003702001949014.
(22) Liu, C.M.; He, H.Y.; Xu, L.; Hua, Z.D. New Qualitative Analysis Strategy for Ilicit Drugs Using Raman Spectroscopy and Characteristic Peaks Method. Drug Test Anal. 2021, 3 (3), 720–728. DOI: 10.1002/dta.2963.
(23) Hargreaves, M.D.; Page, K.; Munshi, T.; Tomsett, R., Lynch, G.; Edwards, H.G. Analysis of Seized Drugs Using Portable Raman Spectroscopy in an Airport Environment—A Proof of Principle Study. J. Raman Spectrosc. 2008, 39 (7), 873–880 (2008).
(24) Milhazes, N.; Martins,P.; Uriarte, E.; Garrido, J.; Calheiros, R.; Marques, M.P.M.; Borges, F. Electrochemical and Spectroscopic Characterisation of Amphetamine-like Drugs: Application to the Screening of 3,4-methylenedioxymethamphetamine (MDMA) and its Synthetic Precursors. Anal. Chim. Acta 2007, 596 (2), 231–241.
(25) Christie, R.; Horan, E.; Fox, J.; O’Donnell, C.; Byrne, J.; McDermott, S.; Power, J.; Kavanagh, P. Discrimination of Cathinone Regioisomers, Sold as ‘Legal Highs’, by Raman Spectroscopy. Drug Test Anal. 2014, 6 (7–8), 651–657. DOI: 10.1002/dta.1518.
Sulaf Assi, Karolina Kielisczyzk, Olivia Wade, and Basel Arafat are with the School of Pharmacy and Biomolecular Sciences at John Moores University, in Liverpool in the United Kingdom. Ismail Abbas is with the Faculty of Science at Lebanese University, in Beirut, Lebanon. Basel Arafat is also with the School of Allied Health at Anglia Ruskin University, in Chelmsford in the United Kingdom. Direct correspondence to: s.assi@ljmu.ac.uk ●

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.