Detection of Trace Elements in High-Purity Sulfuric Acid Using Ultrasonic Nebulization with ICP-AES Detection
Special Issues
This article discusses how coupling an inductively coupled plasma atomic emission spectrometer with an efficient ultrasonic nebulizer improves trace element detection in a 7.5% (v/v) high-purity sulfuric acid matrix.
This article discusses how coupling an inductively coupled plasma atomic emission spectrometer with an efficient ultrasonic nebulizer improves trace element detection in a 7.5% (v/v) high-purity sulfuric acid matrix. Elements of interest include Al, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, Ti, W, Zn, and Zr. A particular goal is to lower instrument detection limits below 0.1 μg/L for K and Na in this matrix.
Water quality is a critical issue in boiler water reactor nuclear power plants (1). Plant water for steam generation is analyzed after passage through sampling filters to check for indications of corrosion. Corrosion particulate matter can cause numerous problems, including reduced water flow rates, increased pump head pressures, and loss of heat-exchange efficiency as a result of solids buildup on metal surfaces. These problems can all lead to expensive component repairs or replacements.
The filters in the water filter packs are periodically removed and digested in a sulfuric acid solution to check for indications of corrosion. Inductively coupled plasma–mass spectrometry (ICP-MS) can be readily used for trace-element analysis of sulfuric acid using cell-based quadrupole (2) or sector-field (3) ICP-MS instruments. However, the use of ICP-MS for this application is expensive and requires careful method development.
This article examines the use of an ultrasonic nebulizer with ICP–atomic emission spectrometry (AES) to improve trace-element detection in a 7.5% high-purity sulfuric acid matrix. The nebulization efficiency of an ultrasonic nebulizer can be up to 10 times higher versus a conventional pneumatic nebulizer as described by Olson, Haas, and Fassel (4) and Bear and Fassel (5). Although non-cleanroom conditions were used, the use of an ultrasonic nebulizer with ICP-AES enabled instrument detection limits for K and Na below 0.10 μg/L.
Experimental
Instrumentation
An iCAP6500 Duo ICP-AES system (Thermo Scientific) was used in this study. A U5000AT+ ultrasonic nebulizer (CETAC Technologies) was coupled to this spectrometer.
Solutions
Reagent blank and standard solutions were prepared from high-purity grade sulfuric acid (Ultrex-II Ultrapure, J.T. Baker). Single-element standards (SCP Science) were used to prepare the working standards and all dilutions were made using 18-MΩ·cm deionized water (Millipore Academic). Solutions were prepared and stored in precleaned fluorinated ethylene propylene (FEP) bottles before analysis.
Ultrasonic Nebulizer: Principle of Operation
In the ultrasonic nebulizer, a peristaltic pump introduces liquid sample across an oscillating piezoelectric transducer; the oscillations disperse the sample into an aerosol that is swept out of a spray chamber by a flow of argon nebulizer gas from the host ICP-AES instrument. The aerosol passes through a heated "u" tube and an electrothermally cooled condenser. Because of the higher efficiency of the transducer, an integrated condenser is necessary to prevent excess sample solvent from destabilizing or extinguishing the plasma. An integrated drain pump removes the condensed sample solvent and any excess sample liquid from the spray chamber. After passing through the condenser, the dried sample aerosol particles are swept by the nebulizer gas to the ICP-AES instrument for analysis. A schematic of the ultrasonic nebulizer is shown in Figure 1.
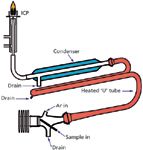
Figure 1: Ultrasonic nebulizer schematic.
Instrument Conditions
Instrument conditions without and with the ultrasonic nebulizer are given in Tables I and II, respectively. An interface kit with dedicated argon-gas-in and sample-out tubing was used to connect the ultrasonic nebulizer to the ICP torch; removal of the standard ICP nebulizer system and installation of the ultrasonic nebulizer takes approximately 5 min. An important operating parameter change was a reduction of nebulizer gas flow rate from 0.60 L/min (standard nebulizer) to 0.50 L/min (ultrasonic nebulizer); all experiments were performed under non-cleanroom conditions.
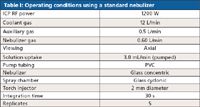
Table I: Operating conditions using a standard nebulizer
Results
Sensitivity Comparison
Net analyte intensities for the standard concentric pneumatic nebulizer and the ultrasonic nebulizer are compared in Table III. Intensities are listed for a 100-μg/L multielement spike in 7.5% (v/v) high-purity sulfuric acid. Analyte intensities with the ultrasonic nebulizer are improved 6.8–17.6 times (average factor of 9.9 times) versus the standard nebulizer.
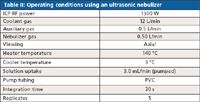
Table II: Operating conditions using an ultrasonic nebulizer
Calibration
A reagent blank and four calibration standards of 20, 50, 100, and 200 μg/L were introduced to the ICP-AES with the standard glass concentric nebulizer and cyclonic spray chamber and then the ultrasonic nebulizer. Instrument detection limits were calculated as three times the standard deviation of the blank concentration; the 100-μg/L standard was run as a check for analyte recoveries.
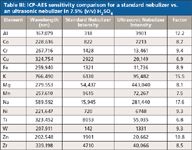
Table III: ICP-AES sensitivity comparison for a standard nebulizer vs. an ultrasonic nebulizer in 7.5% (v/v) H2SO4
Calibration curves for K and Na with the ultrasonic nebulizer are shown in Figures 2a and 2b, respectively. Excellent linearity was obtained, with correlation coefficients of 0.9999 for K at 766.490 nm and Na at 589.592 nm.

Figure 2: ICP-AES calibration in 7.5% (v/v) H2SO4 using ultrasonic nebulization: (a) K, (b) Na.
Analyte Recoveries
Analyte recoveries for the 100-μg/L standard in 7.5% (v/v) high-purity sulfuric acid using both the standard nebulizer and the ultrasonic nebulizer are in a narrow range within ±5% of 100%. The results are listed in Table IV.
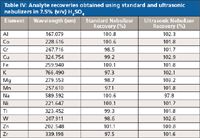
Table IV: Analyte recoveries obtained using standard and ultrasonic nebulizers in 7.5% (v/v) H2SO4
Instrument Detection Limit Comparison
As listed in Table V, the ultrasonic nebulizer lowers instrument detection limits for 13 of the 14 measured elements below 0.1 μg/L (tungsten is 0.24 μg/L), for an average improvement of 5.7× versus the standard nebulizer. The instrument detection limits for K and Na are 0.05 μg/L and 0.03 μg/L, respectively.
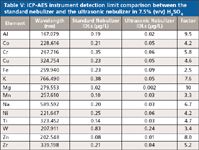
Table V: ICP-AES instrument detection limit comparison between the standard nebulizer and the ultrasonic nebulizer in 7.5% (v/v) H2SO4
Summary
The use of an ultrasonic nebulizer with axial-viewing ICP-AES significantly improves instrument detection limits in a high-purity 7.5% (v/v) sulfuric acid matrix because of the higher nebulization efficiency of the ultrasonic nebulizer transducer. Instrument detection limits using ICP-AES are lowered from a factor of 2.5–10 times for 14 different elements; limits for K and Na are both below the application goal of 0.1 μg/L under non-cleanroom conditions.
Installation of the ultrasonic nebulizer requires removal of the standard nebulizer system with a setup time of 5 min using an interface kit. Modifications to normal ICP-AES operating conditions include a decrease in nebulizer gas flow (from 0.60 L/min to 0.50 L/min) and a modest increase in ICP power (from 1200 W to 1300 W).
References
(1) J.J. Wolff, "Ion-Exchange Resins for Use in Nuclear Power Plants," January 2012. http://www.purolite.com/Customized/Uploads/PuroliteIonExchangeResinsForUseInNuclearPower.pdf.
(2) Agilent ICP-MS Journal Issue 15, Agilent Technologies publication 5988-8983EN, 2003. http://www.chem.agilent.com/Library/flyers/Public/5988_8983EN.pdf.
(3) Thermo Fisher Scientific Application Note 30105, 2006, 2007. https://www.thermo.com/eThermo/CMA/PDFs/Product/productPDF_57328.pdf.
(4) K.W. Olson, W.J. Haas, and V.A. Fassel, Anal. Chem. 49, 623–627 (1977).
(5) B.R. Bear and V.A. Fassel, Spectrochimica Acta, Part B 41B, 1089–1113 (1986).
Fred G. Smith is with CETAC Technologies in Omaha, Nebraska. Please direct correspondence to: fsmith@cetac.com.

Best of the Week: AI and IoT for Pollution Monitoring, High Speed Laser MS
April 25th 2025Top articles published this week include a preview of our upcoming content series for National Space Day, a news story about air quality monitoring, and an announcement from Metrohm about their new Midwest office.
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.