Determination of Contaminants in Beer Using LC–MS/MS and ICP-MS
Special Issues
The authors compare LC–MS/MS methods for quantification of the pesticide glyphosate with and without sample derivatization, and discuss ICP-MS methods for the determination of heavy metals.
The German Beer Purity Law of 1516 makes beer one of the best-analyzed food products with the highest standards regarding quality, freshness, appearance, and flavor. According to this law, beer is allowed to contain hops, malt, yeast, and water as ingredients. Of course, beer also contains major B vitamins, bitter substances, as well as minerals and trace elements (such as Ca, Na, Mg, and Zn) that are important for human nutrition. However, undesirable substances such as pesticides and heavy metals (for instance, Cd, Pb, Hg, Sb, and As) can be found in beer as well, mostly as contaminants in brewing water and grains. In particular, the herbicide glyphosate has to be monitored carefully since it is discussed as a possible carcinogen. The chromatography of glyphosate is challenging because of its high polarity. A well-established method including a derivatization step with 9-fluorenylmethyl chloroformate (FMOC) followed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis is time-consuming and also susceptible to errors. A sample pretreatment without derivatization is desirable because it is faster and cheaper. The use of a triple-quadrupole mass spectrometer optimizes the analytical procedure and establishes a routine method for the analysis of glyphosate in beer. For the determination of elements present in low concentrations, such as As, Se, Pb, Cd, and Zn, inductively coupled plasma-mass spectrometry (ICP-MS) is applied.
Glyphosate is currently one of the most common pesticides used worldwide. In spite of its approval by regulatory bodies all over the world, the concern about its harm to humans and the environment persists. In June 2016, the European Commission decided to extend the license for glyphosate by 18 months, after member states failed to achieve a qualified majority in favor or against the executive’s proposal.
To overcome the challenging chromatography of glyphosate caused by its high polarity, a well-established method can be applied that includes a derivatization step with 9-fluorenylmethyl chloroformate (FMOC) followed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis. Unfortunately, this method is time-consuming, expensive, and susceptible to errors. A further drawback is the fact that not all highly polar pesticides are susceptible to derivatization with FMOC-for example, glufosinate´s metabolite 3-(methylphosphinico)propionic acid (MPPA). Therefore, sample pretreatment without derivatization is desirable, because that approach is quicker and more cost-efficient. Furthermore, it will not be limited to detection of glyphosate, but will allow the simultaneous detection of other highly polar pesticides and their metabolites as well.
In this case study, the quantification of glyphosate, its metabolite aminomethylphosphonic acid (AMPA), glufosinate, and its respective metabolite MPPA based on derivatization are compared to a method without any derivatization. A total of 24 different kinds of beer were tested. The use of high-sensitivity mass spectrometry allowed us to skip a sample concentration step by solid-phase extraction (SPE) for both methods, which additionally reduced time and cost for the analysis.
Sample Preparation for LC–MS
Method and Analytical Conditions
First, 1 mL of methanol was added to 1 mL of beer and the mixture was vortexed. The mixture was then centrifuged for 15 min at 12,000 rpm. Next, 500 µL of the supernatant was used as the underivatized sample. For the derivatization sample, 25 µL of EDTA-borate buffer and 75 µL of FMOC were added to another 500 µL of the supernatant. After a 60-min incubation at 50 °C, the reaction was stopped with 30 µL of 0.2% phosphoric acid. Finally, 125 µL of water was added.
Because derivatized and underivatized samples required different chromatographic conditions, we used an ultrahigh-pressure liquid chromatography (UHPLC) system capable of multiplex analysis (Nexera MX with Dual Stream Technology, Shimadzu) coupled to an MS system (LCMS-8060, Shimadzu) for the application. This approach allowed for the use of a multiplexed method with different columns and LC methods in one batch, thus increasing the throughput and improving the efficiency. The analytical conditions are listed in Table I.
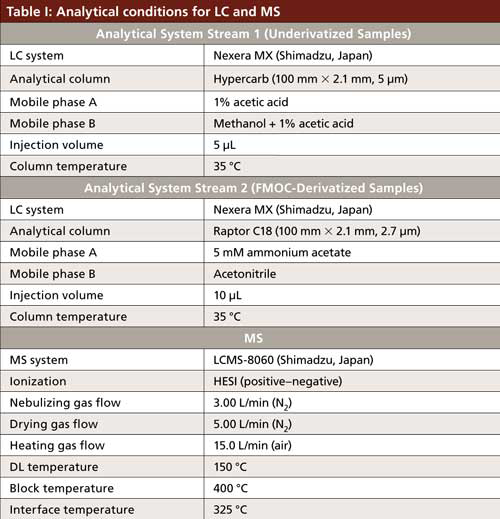
Results of LC–MS Measurements
Two different methods (with and without derivatization) have been established successfully for the quantification of glyphosate and glufosinate in beer, even without the use of internal standards. Glyphosate could be quantified from 5 to 100 ng/mL with both methods while glufosinate could be quantified from 5 to 100 ng/mL with the underivatized method compared to 10–100 ng/mL with the FMOC-derivatization method. With the FMOC-derivatization method, AMPA could be quantified from 5 to 100 ng/mL while MPPA could be quantified from 10 to 100 ng/mL with the underivatized method only. None of these methods are suitable to cope with both metabolites at the same time with sufficient sensitivity.
The calibration curves obtained for the compounds are shown in Figure 1 and the corresponding chromatograms are shown in Figure 2. The analytical results for glyphosate in 24 different beer samples are listed in Table II. Both methods show comparable results. None of the other compounds could be detected in the samples.
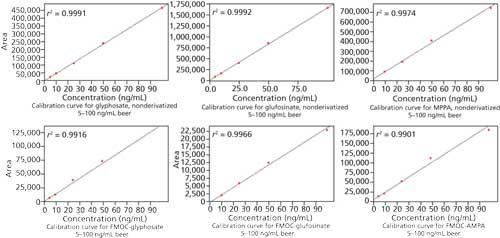
Figure 1: Calibration curves for glyphosate, glufosinate, AMPA, and MPPA determined in duplicate obtained from beer after sample pretreatment. R² was greater than 0.99 for all calibration curves.
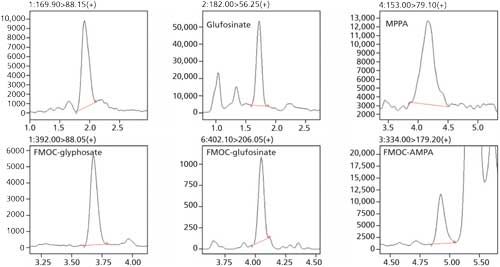
Figure 2: Chromatograms of a 15 ng/mL beer sample.
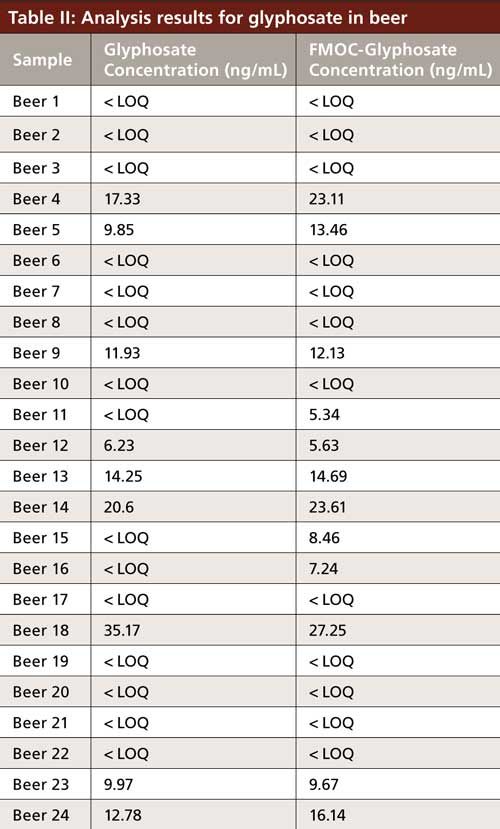
Both methods allow the quantification of all target compounds at or below 0.01 mg/kg (=≤10 ng/mL), which is below the European Union maximum residue levels (MRL) for all studied compounds. The method without sample derivatization shows comparable sensitivity to the FMOC derivatization, but sample pretreatment is much easier and quicker. Glyphosate could be detected in a concentration above the limit of quantitation (LOQ) in more than 30% of the tested commercially available beers. However, even the positive tested samples contained glyphosate in an amount far below the MRL of the raw material (1).
Determination of Heavy Metals Using ICP-MS
The quality standards for beer analysis are defined by the Central European Commission for Brewing Analysis (MEBAK) and the European Brewery Convention (EBC), which is an organization representing the technical and scientific interests of the brewing sector in European countries.
These regulations include the principles and proceedings for the analysis of raw materials, semifinished goods, by-products and finished products, additives and technical supplies, containers, and packaging materials with the aim of standardization, primarily in the field of breweries and malting industries. The technical analysis methods of MEBAK have been published in several application books, including those for the determination of elements such as copper, zinc, sodium, potassium, calcium, anions such as nitrate and sulfite, and organic components such as ethanol, glycerine, and others (2).
Nowadays, the health effects of trace elements and the maximum concentration of trace elements in beers are continuously controlled. The determination of trace metals in beers is relevant because they might be essential or toxic in the human body and they also have an influence on the brewing process. The element distribution, however, shows significant differences based on the natural sources of soil, water, cereal, hops, and yeast as well as anthropogenic sources such as environmental pollution and agricultural treatment by fertilizers, pesticides, and fungicides.
Last but not least, the metal content in beer can be influenced during production, beer processing, conservation, and bottling. This influence is greater than expected because during the beer-making process the raw products and processed products are often in long contact with materials such as stainless steel, copper, glass bottles, and other equipment.
The determination of copper is important because high concentrations are disadvantageous to the colloidal stability and taste of the beer. The same goes for zinc, which is an essential trace element for yeast that influences metabolic processes such as protein synthesis and nucleic acid metabolism. Typical concentration levels of copper and zinc in beer are 0.2 mg/L (3).
Furthermore, the determination of arsenic, antimony, cadmium, and lead is important because those elements are toxic when they are present in beer or the brewing water. The source of these elements in beer and other alcoholic beverages could be the contamination of raw material or technological processes.
Arsenic is released into beer from a filtering material called kieselguhr, or diatomaceous earth, which is used to remove yeast, hops, and other particles and give the beer a crystal clear appearance. Diatomaceous earth consists of fossilized remains of diatoms, a type of hard-shelled algae that lived millions of years ago. Diatomaceous earth finds wide use in filtering beer and wine, and is an ingredient in other products as discussed by Professor Mehmet Coelhan from the Research Center Weihenstephan for Brewing and Food Quality (4).
One more element of concern is antimony. The annual consumption of antimony trioxide in the United States and Europe is approximately 10,000 and 25,000 tons, respectively. The main application is as a flame retardant synergist in combination with halogenated materials. Furthermore, antimony trioxide is used as a catalyst in the production process for polyethylene terephthalate (PET) bottles.
Elevated concentrations of Sb have been found in beverages such as cola drinks and orange juices that are stored in PET bottles, because the Sb migrates from the plastic to the liquid and accumulates in the drinks. The migration process is accelerated in alcoholic beverages. Vodka samples from glass and PET bottles have been compared according to their Sb levels, and it was found that the Sb concentration in vodka from a PET bottle can be as high as 20 µg/L in comparison to less than 1 µg/L in a glass bottle. (5).
The maximum allowable concentration of Sb in drinking water is 5 µg/L. Since the latest development in the beer industry is the introduction of 0.33-, 0.5-, and 1-L PET bottles on supermarket shelves with a variety of beers from European countries, analytical investigations are in process to evaluate the antimony concentrations in beer as well.
High-Sensitivity ICP-MS
For the simultaneous quantitative determination of the inorganic elements in beer, inductively coupled plasma-mass spectrometry (ICP-MS) is the preferred quality control tool. ICP-MS offers high sensitivity (trace detection), a wide dynamic range, and high sample throughput. In this study, we used an ICP-MS system with an octopole collision cell (ICPMS-2030, Shimadzu). Using helium as a collision gas and running in kinetic energy discrimination (KED) mode, this cell suppressed most of the spectroscopic interferences (polyatomic interferences). The efficiency of interference suppressions and the sensitivity are improved by using a cooled cyclonic chamber and a well-controlled torch positioning.
Preparation of Beer Samples and ICP-MS Setup
For this study, samples of two commercially available beers were evaluated. The two beer samples were undiluted and were aspirated after degassing using the measurement parameters listed in Table III. An internal standard solution containing 71Ga, 115In, and 205Tl was added using the automatic internal standard addition kit.
The concentrations of Ni, Cd, Sb, and Pb in the undiluted beer were determined using the calibration curve method. For each studied element, calibration curves were built using four standards in the concentration range from 2 to 10 µg/L. The standards were prepared using a matrix-matched solution containing 5% ethanol.
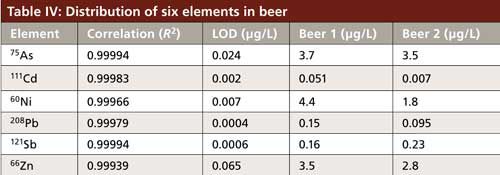
The calibration curves in Figure 3 show that all correlation coefficients are better than 0.999 and low detection limits (LD), which are calculated automatically by the ICP-MS system software (LabSolution-ICPMS) with a 3σ method. Table IV summarizes the results from the study.
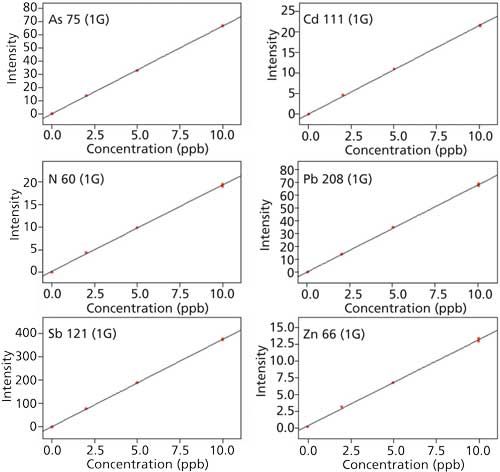
Figure 3: Calibration curves for of 60Ni, 63Sb, 66Zn, 75As, 111Cd, and 208Pb.
Conclusion
Beer is one of the favorite alcoholic beverages in European countries with a statistical per capita consumption of around 68 L. Therefore, the quality is expected to be on a high level and continuous quality control is essential. Still, beer may contain a variety of heavy metals such as arsenic, lead, and cadmium as well as additional undesirable substances like pesticides. High-sensitivity analytical tools such as LC–MS/MS and ICP-MS are best suited to detect low levels of contaminants to permanently ensure the quality of beer.
References
- European Pesticide database: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.CurrentMRL&language=DE
- H. Pfenninger, Brautechnische Analysenmethoden (Band III, Methodensammlung der Mitteleuropäischen Brautechnischen Analysenkommission, 1996).
- J.S. Hough et al., Malting and Brewing Science (Springer US, 1982).
- M. Coelhan et al., Am. Chem. Soc. “Widely Used Filtering Material Adds Arsenic to Beers” (2013), https://www.acs.org/content/acs/en/pressroom/newsreleases/2013/april/widely-used-filtering-material-adds-arsenic-to-beers.html.
- U. Oppermann and J. Schram, GIT Verlag (Wiley-Blackwell, 2011), pp. 102–103.
Uwe Oppermann, Anja Grüning, Ludivine Fromentoux, and Jan Knoop with Shimadzu Europa GmbH, in Duisburg, Germany. Direct correspondence to: uo@shimadzu.eu

NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
New AI Strategy for Mycotoxin Detection in Cereal Grains
April 21st 2025Researchers from Jiangsu University and Zhejiang University of Water Resources and Electric Power have developed a transfer learning approach that significantly enhances the accuracy and adaptability of NIR spectroscopy models for detecting mycotoxins in cereals.