Determination of _α-Dicarbonyls in Wines Using Salting-Out Assisted Liquid–Liquid Extraction
Special Issues
A new methodology for the analysis of three important -dicarbonyls (methylglyoxal, diacetyl, and pentane-2,3-dione) in wines was developed.
A method based on salting-out assisted liquid–liquid extraction for the analysis of α-dicarbonyls in wines was developed. The sample preparation procedure consists of a single step, involving the simultaneous extraction and derivatization of the analytes using an o-phenylenediamine–acetonitrile solution with sodium chloride as the salting-out agent. The obtained organic phase is collected and directly analyzed by liquid chromatography with spectrophotometric detection. The studied α-dicarbonyls were determined in eight wines. The developed methodology substantially reduces the complexity of the sample matrix, which is a very important aspect in routine analysis, especially to ensure long-lasting and reliable functioning of the chromatographic systems, while being a new and attractive methodology for the analysis of α-dicarbonyls.
The α-dicarbonyl compounds have great importance in food quality since they result from enzymatic and chemical processes, namely the Maillard reaction. Among these compounds, diacetyl is the most representative, being known as an important quality marker in food products such as beer, wine, butter, yogurt, and cheese (1–5).
In wines, the major α-dicarbonyls are glyoxal, methylglyoxal, diacetyl, and pentane-2,3-dione, which are formed by malolactic fermentation (6). These compounds have a significant importance in the characteristics of such products because of their sensorial activity, high reactivity with other wine components, and their role in biological processes. The impact of α-dicarbonyls, namely methylglyoxal, in human health has been extensively studied in recent years (7–9).
The determination of α-dicarbonyls is usually performed by chromatographic analysis, either by liquid chromatography (LC) (5,6,10–16) or gas chromatography (GC) (1,6,17,18). The analytes’ extraction is recommended because of their low concentration in the samples and high reactivity of the carbonyl group. The most frequent extraction procedures for GC analysis include solid-phase microextraction (SPME) (1,3,18,19), gas-diffusion extraction (20), and liquid–liquid extraction (LLE) (6,21). For LC analysis it is common to analyze the samples without extraction; α-dicarbonyls are derivatized in-sample and directly injected into the LC system (10,16,22–24), as described in the reference analytical method for α-dicarbonyls analysis of the Organization of Vine and Wine (OIV) (25).
These α-dicarbonyl compounds can be successfully quantified at very low levels in wines (above 0.05 mg/L) (25). However, the use of an extraction step can enhance analyte recovery while avoiding the interference and deleterious effect of several matrix components (such as sugars, lipids, pigments, and so forth) on the chromatographic systems. To address these issues, solid-phase extraction (SPE) (11,13,15) and gas-diffusion extraction (12) procedures were proposed for the analysis of α-dicarbonyls aiming their LC analysis.
In the last decade a well-known but underexplored procedure, called salting-out liquid-liquid extraction (SALLE) (26,27), has been increasingly used in food analysis namely through the QuEChERS (quick, easy, cheap, effective, rugged, and safe) procedure (28). SALLE is a homogeneous liquid–liquid extraction technique in which a water-miscible organic solvent is separated from an aqueous solution by the addition of a salt. Simultaneously, the extraction of analytes to the organic phase is attained when the liquid phases’ separation occurs. In this work, a sample preparation procedure based on SALLE was developed for the analysis of three important α-dicarbonyls (methylglyoxal, diacetyl, and pentane-2,3-dione) in wines.
Materials and Methods
Chemicals and Samples
High-purity water (resistivity not lower than 18.2 MΩ-cm) from a Direct-Q 3 ultraviolet (UV) water purification system (Millipore) was used throughout all the studies. High performance liquid chromatography (HPLC)-grade acetonitrile was from Fisher. All eluents were filtered through a nylon filter (0.45 μm pore size, Whatman) before use.
Methylglyoxal (40% in water), diacetyl (97%), pentane-2,3-dione (97%), hexane-2,3-dione (90%), and o-phenylenediamine (OPDA, 98%) were purchased from Sigma-Aldrich. The salts used (sodium chloride, sodium acetate, and sodium carbonate) were of analytical grade and were also purchased from Sigma-Aldrich.
Stock standard solutions of methylglyoxal (1 g/L) were prepared by diluting the appropriate volume of the commercial reagent in ultrapure water and were stored at 4 °C. Stock standard solutions of diacetyl, pentane-2,3-dione (1 g/L) and hexane-2,3-dione (125 mg/L, internal standard) were prepared in acetonitrile and stored at -20 °C.
The extracting solution containing OPDA (0.5%, m/v) was prepared daily by dissolution of the appropriate amount of the commercial reagent in acetonitrile and stored in the dark.
Wine samples used in this work were of Portuguese origin and were purchased in local markets. The alcohol content in the samples varied between 9.0% and 13.0%, and the pH varied between 3.1 and 3.7.
Extraction Procedure
Wine samples were diluted (2:5, v/v) with acetate buffer (0.2 mol/L, pH 4) and internal standard (hexane-2,3-dione) was added to a final concentration of 0.25 mg/L. In a 10-mL tube, 2 mL of the diluted sample was mixed with 2 mL of OPDA solution and 0.13 g of sodium chloride. After vigorous shaking until an almost complete dissolution of the salt was attained, the mixture was kept in the dark for 1 h at room temperature to simultaneously promote the derivatization of the α-dicarbonyls and their extraction to the acetonitrile phase. The tubes were centrifuged at 6000 rpm for 2 min to improve phase separation and finally an aliquot of the upper phase was collected for HPLC–UV analysis. A simplified scheme of the experimental procedure is shown in Figure 1.
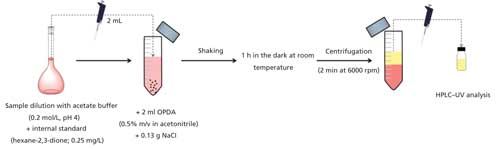
Figure 1: Simplified scheme of the sample preparation procedure.
HPLC–UV Analysis
HPLC–UV analysis was performed using a PerkinElmer S200 chromatographic system with a S200 UV detector. Separation of the derivatized α-dicarbonyls (quinoxalines) was performed using a 250 mm x 4.0 mm, 5-µm dp Phenomenex Gemini C18 column at room temperature. The mobile phase was composed of acetonitrile (mobile-phase A) and 10 mM acetate buffer, pH 4.8 (mobile-phase B). The flow rate was 0.8 mL/min. A gradient was used as follows: 0–5 min 30% A; 5–15 min linear increase of A to 50%; 15–20 min linear decrease of A to 30%; 20–35 min 30% A. The injection volume was 20 µL and UV detection was performed at 315 nm.
Results and Discussion
Effect of the Derivatizing Reagent on the Extraction
In a previous work (26), the application of SALLE to the extraction of α-dicarbonyl compounds from aqueous solutions was studied, opening the possibility to use this technique for beverage analysis. In the present work, three extraction procedures were tested:
- The analytes were initially derivatized in-sample with OPDA for 1 h; the resulting quinoxalines were then extracted by SALLE using acetonitrile and sodium chloride; finally the upper phase was collected and analyzed by HPLC–UV (procedure A in Figure 2);
- α-dicarbonyls were first extracted from the aqueous sample to acetonitrile using sodium chloride; then the upper phase was collected and derivatized with OPDA before HPLC–UV analysis (procedure B in Figure 2); and
- α-dicarbonyls were extracted and derivatized simultaneously using an OPDA solution prepared in acetonitrile using sodium chloride for the phase separation (procedure C in Figure 2).
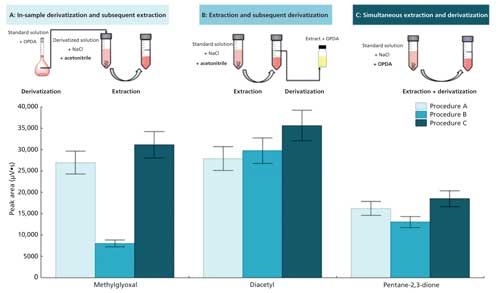
Figure 2: Study of the presence of the derivatizing agent on the extraction using a standard solution of α-dicarbonyls (0.5 mg/L). The three tested experimental procedures (A, B, and C) and the results obtained for each one are depicted. Results are expressed as the mean value of three replicates.
The schemes of the three tested procedures using α-dicarbonyls standard solutions (0.5 mg/L) and the obtained results are shown in Figure 2. The results indicated that the use of the derivatizing agent during the extraction (procedure C) enhanced the recoveries of the analytes, compared to procedure B in which OPDA was absent during the extraction. The presence of OPDA in the extraction medium likely displaced the chemical equilibria toward the acetonitrile phase, favoring the overall extraction of α-dicarbonyl compounds. As expected, the major differences between procedures B and C were observed for the most polar analyte, methylglyoxal (a fourfold increase). The quantity of analytes extracted using procedure C was very similar to that obtained with procedure A. Procedure C (simultaneous extraction and derivatization) was used for the subsequent experiments considering its advantages compared to procedure A in terms of simplicity of the experimental procedure and time saving. The three procedures were also performed with spiked wine samples (0.5 mg/L of each α-dicarbonyl), and the results were similar to those obtained for standard solutions.
Effect of the Salt on the Extraction
The salt used in SALLE as the salting-out agent influenced both the extraction efficiency and the phase separation between acetonitrile and water (26). In this work, three salts were tested (sodium chloride, sodium carbonate, and sodium acetate) using procedure C (Figure 2). Initial experiments with standard solutions (0.25 mg/L) showed that the extraction–derivatization of the analytes was not affected by the salt used. The same experiments were performed using wine (white and red) samples spiked with 0.25 mg/L of each studied carbonyl compound. The results (Figure 3) showed that the determination of α-dicarbonyl compounds in wine samples is influenced by the salt used, suggesting that a chemical modification of the sample matrix is occurring. The first hypothesis put to the test was the possibility of a pH alteration of the sample because of the salt addition (in the case of sodium acetate and sodium carbonate). In fact, it is known that α-dicarbonyls are prone to react with or bind to several wine components such as sulfites (29) and amino acids (30). Since the majority of those binding reactions are pH-dependent, the variation of this parameter can affect the determination of α-dicarbonyl compounds. For this reason, the influence of the wine sample’s pH was studied.
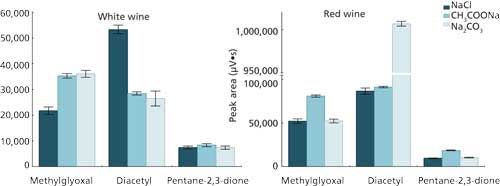
Figure 3: Results obtained from the study of the influence of the salt on the extraction of α-dicarbonyls in spiked white and red wine (0.25 mg/L). NaCl, CH3COONa, and Na2CO3 were used at a concentration of 1 mol/L. Results are expressed as the mean value of three replicates.
Effect of Sample pH
The effect of pH on the extraction of α-dicarbonyls was tested in the pH 2–8 range, using α-dicarbonyls model standard solutions (0.25 mg/L) and wine samples. The results for the extraction of α-dicarbonyls from model standard solutions (data not shown) revealed that peak areas of methylglyoxal were higher at pH above 5 than for pH below 5. The results for diacetyl and pentane-2,3-dione were slightly different showing less interference of pH on the extraction of these compounds. The effect of pH was also studied in diluted wine samples (2:5, v/v) in a buffer solution before the extraction. These studies were performed with the addition of standard solutions to the wine samples and the results (Figure 4) are presented as the concentration determined by the standard additions method. The results showed some influence of pH in the extraction of diacetyl and pentane-2,3-dione in the tested pH range (from pH 2 to 8); for methylglyoxal no significant differences were observed.
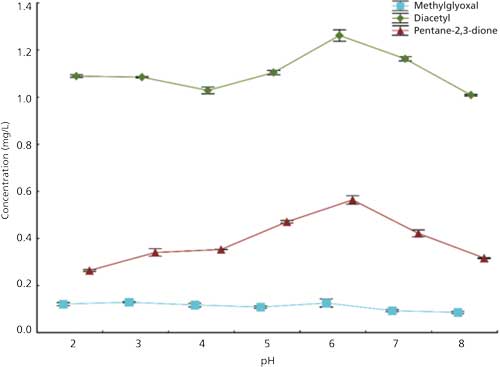
The effect of pH on the extraction of α-dicarbonyls, and other carbonyl compounds, from complex matrices such as wine is still poorly understood. Several discussions about pH influence on the interaction between carbonyls and other wine components, and its impact on the analytical determination of these compounds are available (30). However, a more detailed study of pH-dependent reactions of carbonyls and their implications on the extraction is still scarce. Previous works have already addressed this by studying the pH effect on the extraction of carbonyls considering their interaction with sulfites (29,31,32). However, according to the results obtained in the present work, other interactions are also present and can influence the determination of carbonyl compounds. For this reason, in this work pH control of the sample before the extraction was carefully performed. A pH of 4 was chosen since it is the approximate value of the analyzed samples’ pH.
Methodology Figures of Merit
The linearity of the methodology was studied by the establishment of calibration curves using model standard solutions prepared in buffer solution pH 4 covering α-dicarbonyls concentration ranges normally found in beverages (methylglyoxal, 0.09–0.5 mg/L; diacetyl, 0.2–2.2 mg/L; and pentane-2,3-dione, 0.04–0.2 mg/L). Each concentration level was tested in triplicate. Internal standard (hexane-2,3-dione) was used to compensate any variability on the extraction process. The figures of merit determined for the methodology are summarized in Table I.
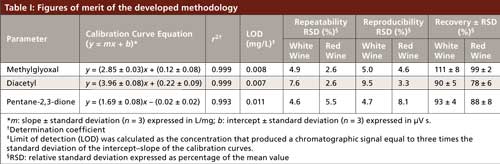
The influence of the sample matrix on the extraction procedure was evaluated by the analysis of two different wine samples (white wine and red wine). Each sample was spiked at four concentration levels (0.1–0.5 mg/L) and analyzed in triplicate. Average values of recoveries ranged from 78% to 111%.
The slopes of the standard addition curves were compared with those of the calibration curves using the Student t-test (99% confidence level). In some cases, significant differences between the slopes were verified. For this reason, the standard additions method was used for the quantification of all α-dicarbonyls in the wine samples.
The repeatability (intraday precision) of the methodology was obtained by the analysis, in the same day, of five replicates of spiked samples. For reproducibility (interday precision), five replicates of the same sample were analyzed in three different days. The relative standard deviation (RSD) values were below 9.5% (Table I) and were considered satisfactory for the levels in which the compounds were found in samples, showing that the method has good precision.
Application of the Methodology to Samples
The developed methodology was applied to the quantification of the studied α-dicarbonyls in eight wines from Portuguese origin. The results are summarized in Figure 5.
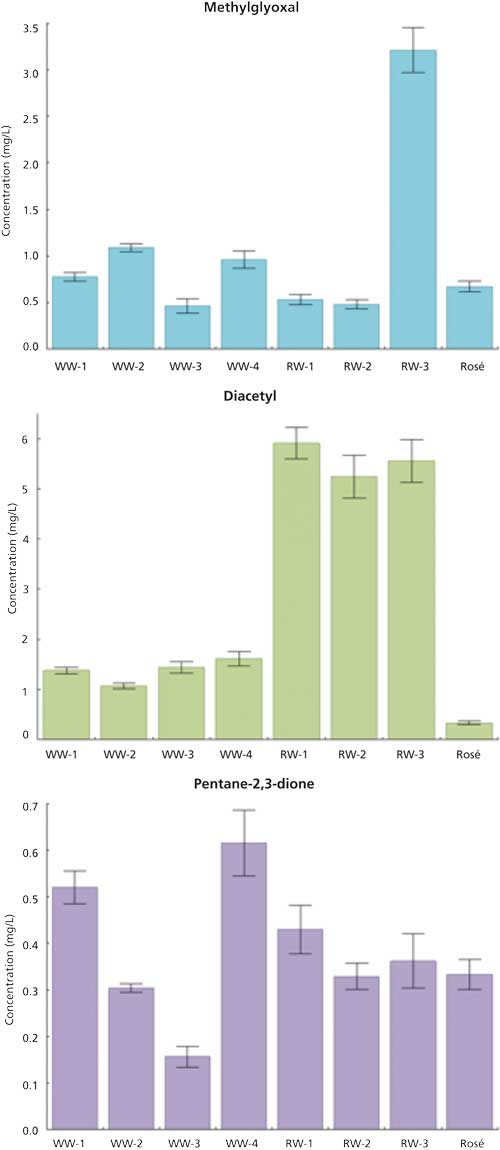
Figure 5: Concentrations of the studied α-dicarbonyls on the analyzed samples (WW = white wine; RW = red wine; Rosé = rosé wine). Experimental conditions used are described in the text and Figure 1.
The methylglyoxal content found in wines was on average 0.7 mg/L, except for one of the red wine samples in which a higher concentration was determined (3.2 ± 0.2 mg/L). The diacetyl concentration was very distinct in white and red wines. The obtained values in white wines (around 1.4 mg/L) were much lower than the concentrations determined in red wines (around 5.6 mg/L). In general, pentane-2,3-dione was the least concentrated α-dicarbonyl found in samples with concentration values ranging from 0.16 ± 0.02 mg/L to 0.62 ± 0.07 mg/L.
Conclusions
A new methodology for the analysis of three important α-dicarbonyls (methylglyoxal, diacetyl, and pentane-2,3-dione) in wines was developed. The sample preparation procedure involves the use of SALLE and combines the extraction and derivatization of the analytes in the same step. The developed methodology proved that it is possible to perform an extraction aided by derivatization, allowing an increase on the analytes recoveries. It was also verified that pH influences the extraction process, becoming a necessary careful control of sample’s pH. The figures of merit of the methodology were evaluated and the studied α-dicarbonyls were determined in eight Portuguese wines.
Acknowledgments
This work received financial support from FCT/MEC through national funds and co-financed by FEDER, under the Partnership Agreement PT2020 - UID/ QUI/50006/2013 -POCI/01/0145/FEDER/007265. IMV (SFRH/BPD/111181/2015) wish to acknowledge FCT for her post-doctoral grant funded by the Portuguese Ministry of Education and Science and by the European Social Fund within the 2014-2020 Strategic Framework.
References
- D. Saison, D. Schutter, F. Delvaux, and F. Delvaux, J. Chromatogr. A1216, 5061–5068 (2009).
- E.J. Bartowsky and P.A. Henschke, Int. J. Food Microbiol. 96, 235–252 (2004).
- Y. Chen, R. Shirey, and L. Sidisky, Chromatographia72, 999–1004 (2010).
- E.J.G. Hernandez, R.G. Estepa, and I.R. Rivas, Food Chem.53, 315–319 (1995).
- G. Zeppa, L. Conterno, and V. Gerbi, J. Agric. Food Chem.49, 2722–2726 (2001).
- G.D. Revel, L. Pripis-Nicolau, J.-C. Barbe, and A. Bertrand, J. Sci. Food Agr.80, 102–108 (2000).
- C.-Y. Lo, W.-T. Hsiao, and X.-Y. Chen, J. Food Sci.76, H90–H96 (2011).
- C.-Y. Lo, S. Li, D. Tan, M.-H. Pan, S. Sang, and C.-T. Ho, Mol. Nutr. Food Res.50, 1118–1128 (2006).
- M.W. Poulsen, R.V. Hedegaard, J.M. Andersen, B. de Courten, S. Bügel, J. Nielsen, L.H. Skibsted, and L.O. Dragsted, Food Chem. Toxicol. 60, 10–37 (2013).
- P. Li, Y. Zhu, S. He, J. Fan, Q. Hu, and Y. Cao, J. Agric. Food Chem. 60, 3013–3019 (2012).
- A. Barros, J.A. Rodrigues, P.J. Almeida, and M.T. Oliva-Teles, J. Liq. Chromatogr. Relat. Technol.22, 2061–2069 (1999).
- J.G. Pacheco, I.M. Valente, L.M. Gonçalves, P.J. Magalhães, J.A. Rodrigues, and A.A. Barros, Talanta81, 372–376 (2010).
- S.L. McCarthy, J. Am. Soc. Brew. Chem. 53, 178–181 (1995).
- W. Bednarski, L. Jedrychowski, E.G. Hammond, and Z.L. Nikolov, J. Dairy Sci. 72, 2474–2477 (1989).
- A.C.D.S. Ferreira, S. Reis, C. Rodrigues, C. Oliveira, and P.G. De Pinho, J. Food Sci.72, S314–S318 (2007).
- M. Yamaguchi, J. Ishida, Z.X. Xuan, A. Nakamura, and T. Yoshitake, J. Liq. Chromatogr.17, 203–211 (1994).
- OIV, Method OIV-MA-AS315-21. Compendium of International Methods of Wine and Must Analysis, International Organisation of Vine and Wine. Paris, France, 2013.
- M.-l. Bao, F. Pantani, O. Griffini, D. Burrini, D. Santianni, and K. Barbieri, J. Chromatogr. A809, 75–87 (1998).
- J. Beránek and A. Kubátová, J. Chromatogr. A1209, 44–54 (2008).
- C. Mathis, M.N. Pons, J.M. Engasser, and M. Lenoel, Anal. Chim. Acta 279, 59–66 (1993).
- OIV, Method OIV-MA-AS315-21. Compendium of International Methods of Wine and Must Analysis, International Organisation of Vine and Wine. Paris, France, 2013.
- J. Degen, M. Hellwig, and T. Henle, J. Agric. Food Chem. 60, 7071–7079 (2012).
- S. Gensberger, S. Mittelmaier, M. Glomb, and M. Pischetsrieder, Anal. Bioanal. Chem.403, 2923–2931 (2012).
- M.I. Rodríguez-Cáceres, M. Palomino-Vasco, N. Mora-Diez, and M.I. Acedo-Valenzuela, Food Chem.187, 159–165 (2015).
- OIV, Method OIV-MA-AS315-20. Compendium of International Methods of Wine and Must Analysis, International Organisation of Vine and Wine. Paris, France, 2013.
- I.M. Valente, L.M. Gonçalves, and J.A. Rodrigues, J. Chromatogr.A1308, 58–62 (2013).
- R.E. Majors, LCGC North Am. 27(7), 526–533 (2009).
- M. Anastassiades, S.J. Lehotay, D. Štajnbaher, and F.J. Schenck, J. AOAC Int. 86, 412–431 (2003).
- R.M. Ramos, J.G. Pacheco, L.M. Gonçalves, I.M. Valente, J.A. Rodrigues, and A.A. Barros, Food Control24, 220–224 (2012).
- L. Pripis-Nicolau, G. de Revel, A. Bertrand, and A. Maujean, J. Agric. Food Chem. 48, 3761–3766 (2000).
- L.C. Azevedo, M.M. Reis, L.F. Motta, G.O. Rocha, L.A. Silva, and J.B. de Andrade, J. Agric. Food Chem. 55, 8670–8680 (2007).
- M. Cruz, I. Valente, L. Gonçalves, J. Rodrigues, and A. Barros, Anal. Bioanal. Chem.403, 1031–1037 (2012).
Inês Maria Valente is with the REQUIMTE/LAQV - Departamento de Química e Bioquímica, Faculdade de Ciências, and the Departamento de Clínicas Veterinárias, Instituto de Ciências Biomédicas Abel Salazar at the Universidade do Porto in Porto, Portugal. José António Rodrigues is with the Departamento de Química e Bioquímica, Faculdade de Ciências at the Universidade do Porto. Direct correspondence to: ines.valente@fc.up.pt

NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
New AI Strategy for Mycotoxin Detection in Cereal Grains
April 21st 2025Researchers from Jiangsu University and Zhejiang University of Water Resources and Electric Power have developed a transfer learning approach that significantly enhances the accuracy and adaptability of NIR spectroscopy models for detecting mycotoxins in cereals.