Determination of Sulfuric Acid Effects on Degradation and Structural Changes of Gelatin Using Fourier-Transform Infrared Spectroscopy and Peak Deconvolution Analysis
The present research was aimed to investigate the effects of sulfuric acid on the structures of gelatin polypeptides. Gelatin samples were immersed in 0.5 M sulfuric acid solution for different periods of 15, 30, 60, 120, 240, 480, 960, and 1920 s, with possible structural changes analyzed by Fourier-transform infrared spectroscopy (FT-IR). Spectra at amide I and II regions were scrutinized using the Gaussian deconvolution method for the resulting changes in the protein secondary structure. The hydrolysis process initially led to a decrease in the α-helix chain and an increase in random coil and β-sheet structures. An equilibrium was formed in degradation and these structures were sequentially turned on each other. Results revealed a correlation between the peak intensity changes of these conformations, so that the degradation process could be observed in the conversion of α-helix to random coil and β-sheet structures and vice versa, indicating the oxidation and expansion of protein structure at the onset of the degradation process.
Protein-based materials account for a significant portion of biostructures, food, and even historical and cultural objects, including gelatin, a protein derivative excessively used in various fields. It is an abundantly available polypeptide derived from collagen hydrolysis in exposure to acid (type A) or alkali (type B) (1–3). The presence of multiple functional groups has enabled gelatin to chemically form crosslinks (4). Despite the fact that the structure of collagen is hydrolyzed in the production process, molecules of gelatin still retain their primary structure (3,5). A great deal of structural diversity with a variety of properties can be found in this material, which depends entirely on parameters such as the source of collagen, type of collagen, method of collagen conversion to gelatin, and the age of the animal (1,5). Due to the events of the secondary and tertiary structures and the triple helical arrangement, gelatin forms physical hydrogels (6), resulting in extensive applications in historical objects and their conservation.
Gelatin, as a complex collagen-based biopolymer, is widely applied as an adhesive (animal glue). It has been hired to preserve and restore cultural and historical artifacts and objects such as paintings, furniture, and books, as well as used as a stabilizer, binding medium, and lining of canvas paintings (7,8). In addition, gelatin is sometimes utilized in the treatment of acidic-degraded historical protein-based objects, which are generally exposed to sulfuric acid (9,10). Historical protein artifacts, especially leathers tanned with condensed tannins (for example, Mimosa tannin), are very vulnerable to sulfur dioxide, which is transformed into sulfuric acid (the most aggressive agent of acid deterioration in vegetable tanned leather) in the presence of moisture (11–13). Advanced acidic deterioration of vegetable-tanned leather, red rot, shows lower strength, pH and hydrothermal stability, has a powdery surface, and displays a loss of the grain layer (10,14). Also, chemical deterioration may lead to the gelatinization of collagen (15).
However, the structure of gelatin is very sensitive to various environmental parameters (16). The gelatin-based materials are characterized by poor mechanical properties, thermal instability, and relatively short degradation times (1). Nonetheless, gelatin degradation processes have been less studied than other conservation materials and historical objects, despite the fact that the analysis of gelatin degradation processes can provide appropriate therapeutic solutions, and help to understand the damage caused to it and the selection of this substance for specific applications.
In the gelatinization process, the triple helical structure is destroyed, but molecular composition changes slightly (1). Hydrolysis and subsequent conversion of collagen to gelatin target mainly tertiary and quaternary structures of collagen, while the primary structure remains almost unchanged (5). However, many of the properties of gelatin can be attributed to secondary structure conformations, mainly consisting of α-helix chains, β-sheets, and random coils that appear depending on temperature, solvent, and pH (17). Gelatin is simply obtained by converting the triple helix of collagen into random coils (18). During gelatin extraction, the triple helices are first cleaved into a single-stranded α-helix (3,5,19). However, during the subsequent gelatinization and drying process of the gelatin, these triple helices are subsequently formed (7,19). These helices act as physical crosslinks in the gelatin, resulting in a three-dimensional lattice structure. The extent of such helices is of the physical properties of gelatin-based adhesives, accounting for their mechanical and thermal performance, which can be influenced by various factors, such as environmental conditions, collagen origin, preparation process, and gelatin concentration (7,20).
Protein studies routinely focus on secondary structure conformations (21). The various conformations that may be found or formed in the gelatin secondary structure commonly include α-helix chains, β-sheets, turns, and random coils (21–23). The conformation stability generally depends on the intrachain forces between the amino acid monomers along the polypeptide chain (5). Numerous factors such as heat, moisture, or many chemical solutions, including acids involved in the degradation process of many historical objects, have the potential to change, transform, and even stabilize these conformations (5,24). One of the methods proposed to explore these conformations in the gelatin structure is Fourier transform infrared spectroscopy (FT-IR). In the FT-IR spectra, peak deconvolution and fitting can be applied to expanded bands due to different compounds with identical vibrational energy in α-helices, β-sheets, and random coils (25). Accordingly, this study aimed to evaluate the effect of the sulfuric acid solution on the conformational changes and degradation of gelatin using FT-IR spectros- copy and peak deconvolution analysis.
Experimental Section
In this study, the changes in polypeptide chains were investigated using gelatin (Merck) as a film with a thickness of about 200–300 μm on a glass slide. A 20% gelatin solution was smeared on the slide, and then examined after being dried at an ambient temperature for 72 h. Polypeptide chain degradation was assessed using 0.5 M sulfuric acid (Merck) due to both hydrolyzing and oxidizing behaviors. The samples were immersed in the acidic solution for 32 min at various intervals of 15, 30, 60, 120, 240, 480, 960, and 1920 s. Then, the samples were placed in the laboratory condition for 48 h to establish moisture balance with the environment, followed by recording the FT-IR spectra using the Jasco 680Plus spectrometer (Jasco Inc.), based on the KBr pellet method, during 64 scans with a resolution of 2 cm-1 ranged from 400 to 4000 cm-1. The resulting spectra were analyzed by OriginPro 2021 software. The position of the peaks was determined after baseline correction in the range of 1485–1800 cm-1 (corresponding to the vibrations of amide I and II) using the second-order derivative. The Gaussian function was used to determine the deconvolution and fitting of the peaks, and the structural changes were evaluated based on the resulting peaks.
Results and Discussion
Amides I and II are the most prominent regions in the FT-IR spectra of proteins, whose vibrations are visible around 1650 and 1550 cm-1, respectively (26). Previous studies have shown that examining the peak intensity ratio of amide I to II and the distance between these two peaks provides information about the intensity of hydrolysis and denaturation of the protein, respectively. Increased acid hydrolysis in the protein intensifies the ratio of amide I to II, which is about 1.25:1.30 in the normal state, one of the reasons for which can be attributed to hydraulic fracturing and increased OH bending vibrations of about 1650 cm-1, resulting in the elevated intensity of the amide I absorption band. Protein denaturation increases the distance between the two peaks, which is about 90–100 cm-1 normally, although some studies have shown the opposite (27–29).
However, the vibrations ranging 1500–1800 cm-1 also provide significant information about the protein secondary structure that mainly consists of α-helices, β-sheets, and random coils (Figure 1) (22). These structures are capable of evaluating in this range through peak deconvolution analysis. Therefore, peak deconvolution analysis of the spectra was performed in the range of amide I and II vibrations using the Gaussian function, as shown in Figure 2. The position of the peaks was identified using the second-order derivative of the spectra. In these spectra and range of amide I, the identified peaks at about 1630, 1660, and 1690 cm-1 are attributed to random coils (25), α-helices (25,30), and β-sheets (31), respectively.
FIGURE 1: The main secondary structures of protein, the alpha-helix and beta-sheet.
FIGURE 2: Peak deconvolution analysis based on second-order derivative signals and curve fitting in the range of 1485–1800 cm-1 for the control sample and the samples immersed in 0.5 M sulfuric acid for 15 to 1920 sec (Seconds noted in upper left-hand corner of each subfigure as T:s).
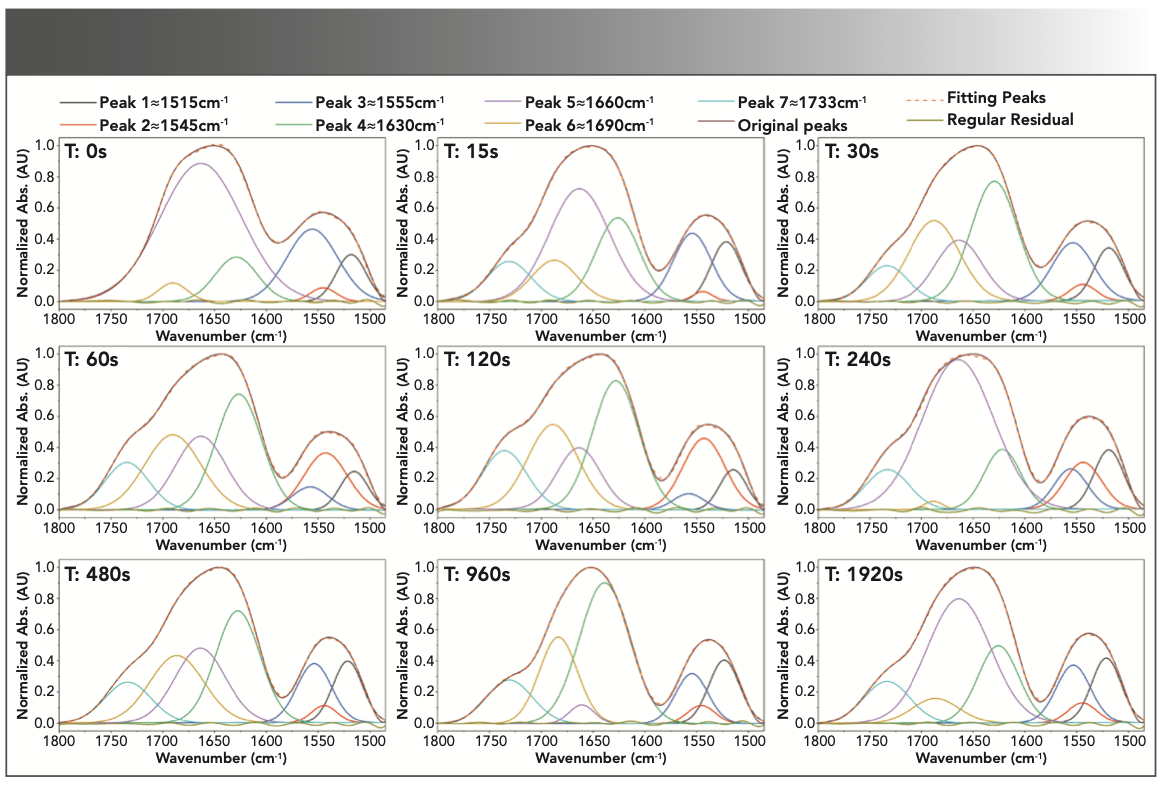
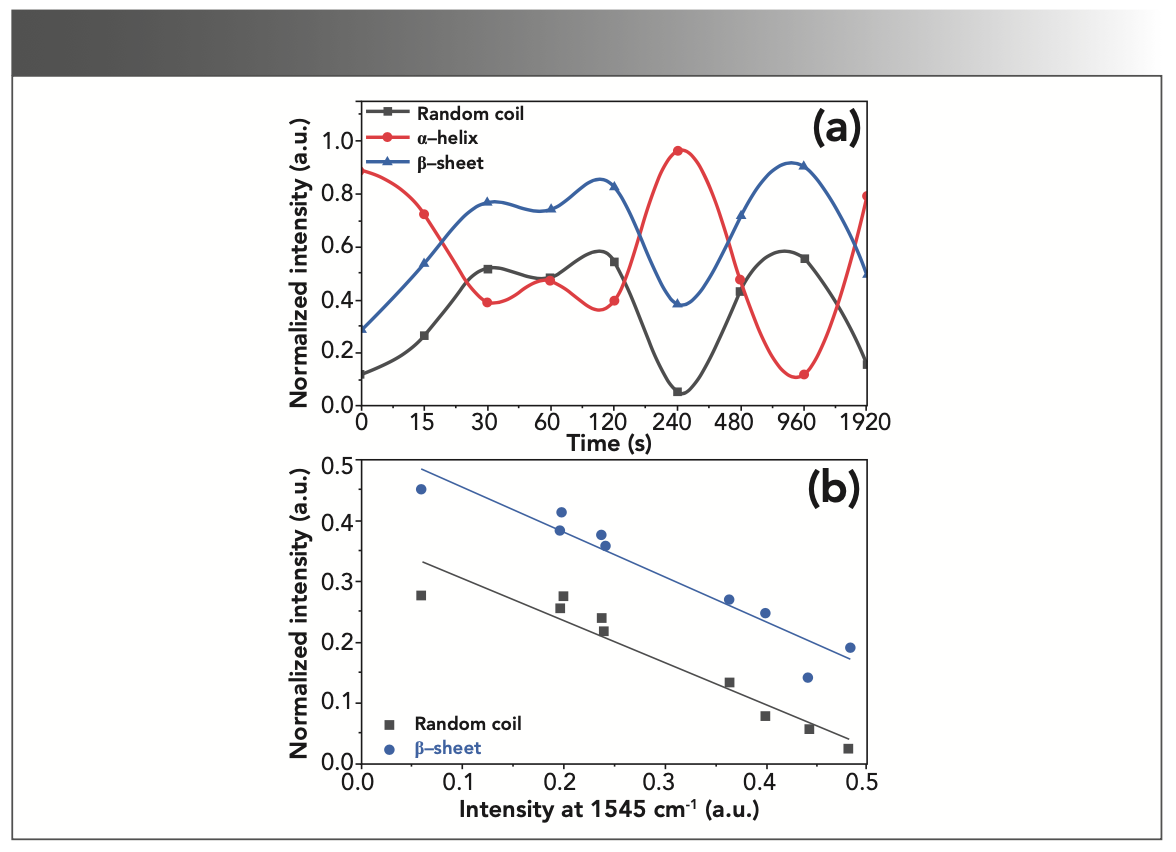
The protein denaturation normally converts α-helix to random coil (7). In collagenous materials, this mainly leads to gelatinization and degradation of the structure. However, the structure of gelatin also contains α-helices, which usually appear at 1655 cm-1, the extent of which is likely to change as a result of gelatin degradation. There are several reasons for protein degradation, the most important of which is heat. A triple helix generally loses its conformation when exposed to heat, and becomes a random coil. Similar degradation can be induced by mineral acids such as H2SO4 and HCl (24). However, the conversion of α-helix to β-sheet is another exchange due to changes in the secondary structure of the protein (32). It should be noted that β-sheet is commonly slightly more stable than α-helix (33). Accord- ingly, the intensity of deconvoluted peaks of the random coil, α-helix, and β-sheet was estimated to be around 1630, 1660, and 1690 cm-1, respectively, based on the intervals of immersion in the acid solution. As shown in Figure 3, the absorption band intensity decreased for α-helix and increased for random coil and β-sheet during the initial 120 s, highlighting the conversion of an α-helix to β-sheet and random coil due to degradation. But after 120 s, we can observe the alternating conversion of these two structures to α-helix, and vice versa. Regardless of the immersion time, these transformations, or the inverse relationship of α-helix and β-sheet intensities, as well as α-helix and random coil, can be seen in Figure 3.
FIGURE 3: (a) Vibration intensity changes around 1630, 1660, and 1690 cm-1 corresponding to the random coil, α-helix, and β-sheet, respectively; (b) Analyzing the intensity changes of random coil and β-sheet compared to α-helix in the amide I region, implying a significant inverse relationship.
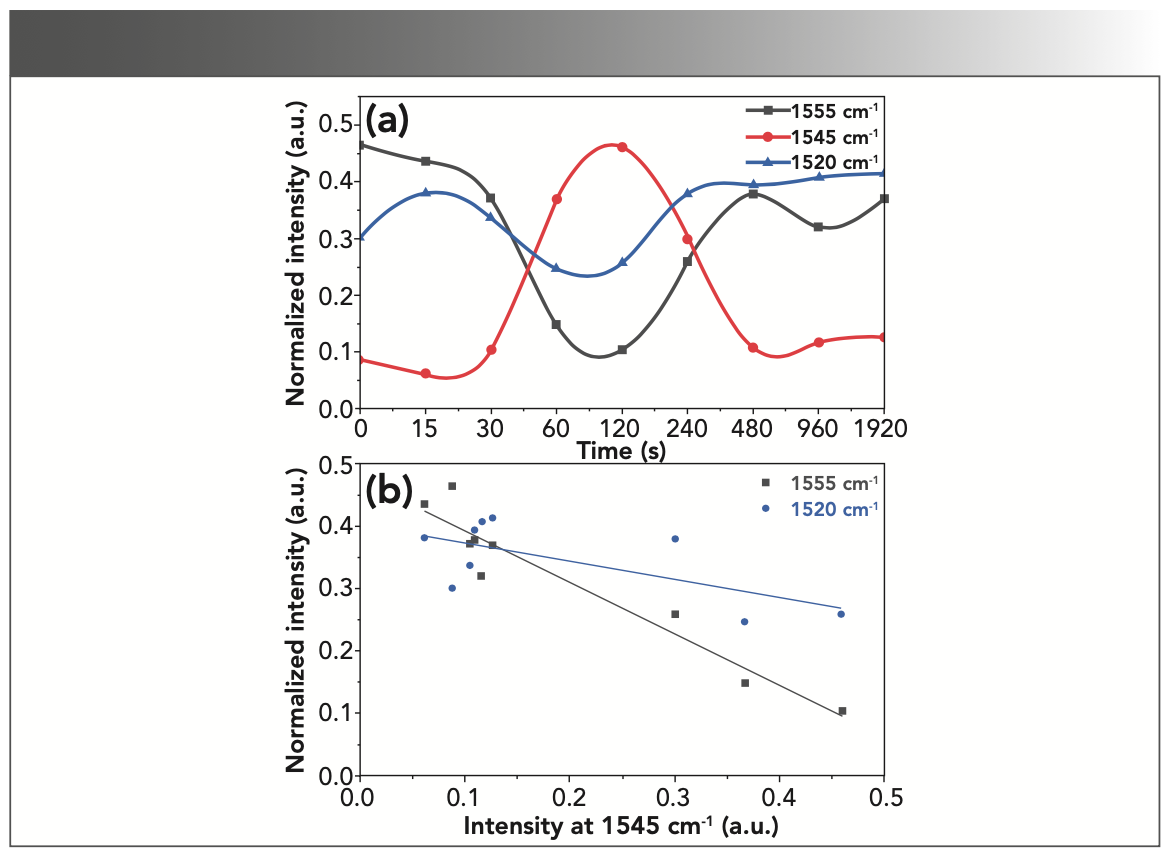
In addition to the vibrations of the amide I region, these successive exchanges of secondary structure conformations can also be observed in the amide II region. The peaks of this range have been attributed to various structures. The peaks at 1520, 1545, and 1555 cm-1 generally correspond to β-sheet, α-helix, and random coil (and in some cases, to turns), respectively (34–37). As in Figure 4a, decreasing or increasing the α-helix at 1545 cm-1 causes an inverse change in the intensities of random coil and β-sheet, 1555 and 1520 cm-1. However, as in Figure 4b, the correlation of the changes is much lower when compared to the amide I absorption bands shown in Figure 3. Accordingly, the amide I absorption bands provide a more suitable range to explore the changes in the protein secondary structure.
FIGURE 4: (a) Vibration intensity changes around 1520, 1545, and 1555 cm-1 corresponding to β-sheet, α-helix, and random coil (or turns), respectively; (b) Analyzing the intensity changes of random coil and β-sheet compared to α-helix in the amide II region, implying a significant inverse relationship.
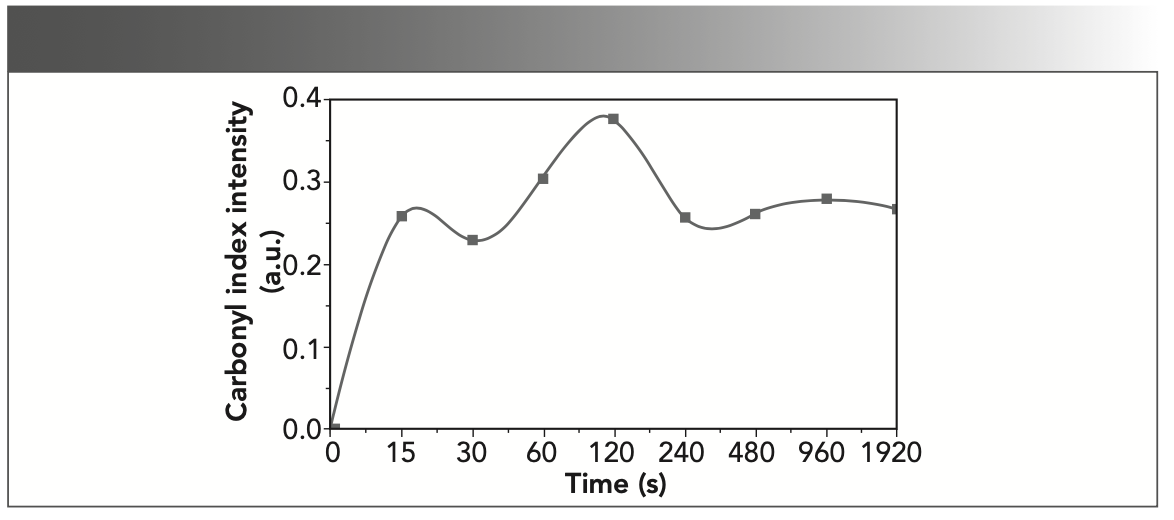
Oxidation is another key process of degradation in protein structures. Sulfuric acid, in addition to being a strong acid and hydrolyzing most organic compounds, also acts as a strong and destructive oxidizing agent (38). Therefore, in addition to the damage caused by hydrolysis, oxidation changes in gelatin can also be examined. During the oxidation processes, the polypeptides are split into shorter fragments, consisting of acid amides or keto acid derivatives (39). The oxidation of polypeptide chains leads to the formation and elevation of carbonyl compounds, resulting in the appearance of a band at 1700–1750 cm-1. Analysis of the relative intensity of this band can contribute to a better understanding of the process and extent of degradation in protein structures (40). However, the overlap of this absorption band with the wide amide band I does not allow direct investigation, and the peak deconvolution analysis provides more appropriate information. As in Figure 2, peak 7 at 1735 cm-1 corresponding to carbonyl vibrations is absent in the reference sample, but this peak is evident after the first exposure to the acidic solution. The intensity of this peak (Figure 5) has been increased up to 120 s, which indicates the intensification of gelatin oxidation. However, after this period, a relative equilibrium is evident at the level of the carbonyl groups.
FIGURE 5: Changes in absorption band intensity at about 1735 cm-1 corre- sponding to carbonyl vibrations as an oxidation index.
Conclusions
The present study investigated the effect of 0.5 M sulfuric acid on the structural changes of gelatin using peak deconvolution analysis and FT-IR spectra. According to the results, the acid causes some changes in the secondary structure of the protein. During the degradation process, the α-helix chain is transformed into random coils and β-sheets, as evidenced by changes in the intensity of peaks detected in the amide I region. These changes go through a straightforward process for up to 120 s, and then reach an oscillating equilibrium in peak intensity due to the intermittent conversions of the structures to each other. In addition to the amide I region, information on changes in the protein secondary structure, including α-helix, β-sheet, and random coil (or turns), can also be explored in the amide II region; decreasing α-helix increases β-sheet and random coil (or turns), and vice versa. The results from the amide I region appear to be more appropriate than those from the amide II region.
Another change due to the effect of sulfuric acid on the gelatin structure was the oxidation of the polypeptide chain, which can be observed by increasing the intensity of the carbonyl adsorption band by about 1735 cm-1 which was intensified to 120 s of immersion. As for this, due to the overlapping of the amide I peak with the carbonyl vibration, the peak deconvolution analysis provided an admirable opportunity to explore changes in carbonyl peak intensity.
Declarations
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
The author declares that he has no competing interests.
Funding
This research received no external funding.
Acknowledgments
Not applicable.
References
(1) Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14 (2), 396. DOI: 10.3390/ma14020396
(2) Stevens, P. Gelatine. In Food Stabilisers, Thickeners and Gelling Agents, Imeson, A. Ed.; Wiley-Blackwell, 2009; pp 116–144.
(3) Derkach, S. R.; Kuchina, Y. A.; Baryshnikov, A. V.; Kolotova, D. S.; Voron’ko, N. G. Tailoring Cod Gelatin Structure and Physical Properties with Acid and Alkaline Extraction. Polymers 2019, 11 (10), 1724. DOI: 10.3390/polym11101724
(4) De France, K. J.; D’Emilio, E.; Cranston, E. D.; Geiger, T.; Nyström, G. Dual Physically and Chemically Crosslinked Regenerated Cellulose—Gelatin Composite Hydrogels Towards Art Restoration. Carbohydr. Polym. 2020, 234, 115885. DOI: 10.1016/j.carbpol.2020.115885
(5) Sajkiewicz, P.; Kołbuk, D. Electrospinning of Gelatin for Tissue Engineering—Molecular Conformation as One of the Overlooked Problems. J. Biomater. Sci. Polym. Ed. 2014, 25 (18), 2009–2022. DOI: 10.1080/09205063.2014.975392
(6) Rebers, L.; Granse, T.; Tovar, G. E. M.; Southan, A.; Borchers, K. Physical Interactions Strengthen Chemical Gelatin Methacryloyl Gels. Gels 2019, 5 (1), 4. DOI: 10.3390/gels5010004
(7) Mosleh, Y.; de Zeeuw, W.; Nijemeisland, M.; Bijleveld, J. C.; van Duin, P.; Poulis, J. A. The Structure–Property Correlations in Dry Gelatin Adhesive Films. Adv. Eng. Mater. 2021, 23 (1), 2000716. DOI: 10.1002/adem.202000716
(8) Casoli, A.; Isca, C.; De Iasio, S.; Botti, L.; Iannuccelli, S.; Residori, L.; Ruggiero, D.; Sotgiu, S. Analytical Evaluation, by GC/MS, of Gelatine Removal from Ancient Papers Induced by Wet Cleaning: A Comparison Between Immersion Treatment and Application of Rigid Gellan Gum Gel. Microchem. J. 2014, 117, 61–67. DOI: 10.1016/j.microc.2014.06.011
(9) van Soest, H. A. B.; Stambolov, T.; Hallebeek, P. B. Conservation of Leather. Stud. Conserv. 1984, 29 (1), 21–31. DOI: 10.1179/sic.1984.29.1.21
(10) Mahony, C. C. Evaluation of Consolidants for the Treatment of Red Rot on Vegetable Tanned Leather: The Search for a Natural Material Alternative. University of California, Los Angeles, 2014.
(11) Strzelczyk, A. B.; Kuroczkin, J.; Krumbein, W. E. Studies on the Microbial Degradation of Ancient Leather Bookbindings: Part I. Int. Biodeterior. 1987, 23 (1), 3–27. DOI: 10.1016/0265-3036(87)90039-X
(12) Dif, K.; Pepe, C.; Peduzzi, J.; Lavedrine, B.; Chahine, C. An Approach of a Study of the Interaction Between Collagen and Sulphur Dioxide by Using ESI and MALDI-TOFMS. J. Cult. Herit. 2002, 3 (4), 317-323. DOI: 10.1016/S1296-2074(02)01241-4
(13) Koochakzaei, A.; Mallakpour, S.; Ahmadi, H. Performance Evaluation of Hybrid Formulations Consisting of Antioxidant and Crosslinking Agents for the Treatment of Acid Degradation in Historic Vegetable-Tanned Leathers. J. Am. Leather Chem. Assoc. 2022, 117 (8), 330–337.
(14) Lama, A.; Antunes, A. P. M.; Covington, A. D.; Guthrie-Strachan, J.; Fletcher, Y. Use of Aluminium Alkoxide and Oxazolidine II to Treat Acid-Deteriorated Historic Leather. J. Inst. Conserv. 2015, 38 (2), 172–187. DOI: 10.1080/19455224.2015.1071713
(15) Larsen, R.; Sommer, D. V. P.; Axelsson, K. M.; Frank, S. Transformation of Collagen into Gelatine in Historical Leather and Parchment Caused by Natural Deterioration and Moist Treatment. In Proceedings of the ICOM-CC, Leather and Related Materials Working Group. Interim Meeting, 2012; Vol. 31, pp 61–68.
(16) Duconseille, A.; Wien, F.; Audonnet, F.; Traore, A.; Refregiers, M.; Astruc, T.; Santé-Lhoutellier, V. The Effect of Origin of the Gelatine and Ageing on the Secondary Structure and Water Dissolution. Food Hydrocoll. 2017, 66, 378–388. DOI: 10.1016/j.foodhyd.2016.12.005
(17) Furlan, P. Y.; Scott, S. A.; Peaslee, M. H. FTIR-ATR Study of pH Effects on Egg Albumin Secondary Structure. Spectrosc. Lett. 2007, 40 (3), 475–482. DOI: 10.1080/00387010701295950
(18) Ahmad, T.; Ismail, A.; Ahmad, S. A.; Khalil, K. A.; Teik Kee, L.; Awad, E. A.; Sazili, A. Q. Physicochemical Characteristics and Molecular Structures of Gelatin Extracted from Bovine Skin: Effects of Actinidin and Papain Enzymes Pretreatment. Int. J. Food Prop. 2019, 22 (1), 138–153. DOI: 10.1080/10942912.2019.1576731
(19) Schellmann, N. C. Animal Glues: A Review of Their Key Properties Relevant to Conservation. Stud. Conserv. 2007, 52 (sup1), 55–66. DOI: 10.1179/sic.2007.52.Supplement-1.55
(20) Bigi, A.; Panzavolta, S.; Rubini, K. Relationship Between Triple-Helix Content and Mechanical Properties of Gelatin Films. Biomaterials 2004, 25 (25), 5675–5680. DOI: 10.1016/j.biomaterials.2004.01.033
(21) Pelton, J. T.; McLean, L. R. Spectroscopic Methods for Analysis of Protein Secondary Structure. Anal. Biochem. 2000, 277 (2), 167–176. DOI: 10.1006/abio.1999.4320
(22) Gupte, T. M.; Ritt, M.; Sivaramakrishnan, S. Chapter Seven - ER/K-link—Leveraging a native protein linker to probe dynamic cellular interactions. In Methods in Enzymology, Merkx, M. Ed.; Vol. 647; Academic Press, 2021; pp 173–208.
(23) Berg, J. M.; Stryer, L.; Tymoczko, J. L. Secondary Structure: Polypeptide Chains Can Fold Into Regular Structures Such as the Alpha Helix, the Beta Sheet, and Turns and Loops. In Biochemistry, 5th ed.; W. H. Freeman, 2002.
(24) Wagermaier, W.; Fratzl, P. Collagen. In Reference Module in Materials Science and Materials Engineering, Elsevier, 2016.
(25) Boyatzis, S. C.; Velivasaki, G.; Malea, E. A Study of the Deterioration of Aged Parchment Marked with Laboratory Iron Gall Inks Using FTIR-ATR Spectroscopy and Micro Hot Table. Heritage Sci. 2016, 4 (1), 13. DOI: 10.1186/s40494-016-0083-4
(26) Barth, A. Infrared Spectroscopy of Proteins. Biochimica et Biophysica Acta (BBA) - Bioenergetics 2007, 1767 (9), 1073–1101. DOI: 10.1016/j.bba- bio.2007.06.004
(27) Koochakzaei, A.; Ahmadi, H.; Mallakpour, S. An Experimental Comparative Study of the Effect of Skin Type on the Stability of Vegetable Leather under Acidic Condition. J. Am. Leather Chem. Assoc. 2018, 113 (11), 345–351.
(28) Koochakzaei, A.; Ahmadi, H.; Achachluei, M. An Experimental Comparative Study on Silicone Oil and Polyethylene Glycol as Dry Leather Treatments. J. Am. Leather Chem. Assoc. 2016, 111 (10), 377–382.
(29) Koochakzaei, A.; Ahmadi, H.; Achachlouei, M. M. Performance Evaluation of Dimethyl Silicone Oil as Archaeological Dry Leather Lubricant. J. Am. Leather Chem. Assoc. 2020, 115 (4), 140–144.
(30) Khalid, I.; Sharkh, S.; Samamarh, H.; Alfaqeeh, R.; Abuteir, M.; Darwish, S. Spectroscopic Characterization of the Interaction between Dopamine and Human Serum Albumin. Open J. Biophys. 2019, 9 (2), 110–130. DOI: 10.4236/ojbiphy.2019.92009
(31) Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39 (8), 549–559. DOI: 10.1111/j.1745-7270.2007.00320.x
(32) Calabrò, E.; Magazù, S. Fourier Self-Deconvolution Analysis of β-Sheet Contents in the Amide I Region of Hemoglobin Aqueous Solutions under Exposure to 900 MHz Microwaves and Bioprotective Effectiveness of Sugar and Salt Solutions. Spectrosc. Lett. 2015, 48 (10), 741–747. DOI: 10.1080/00387010.2015.1011278
(33) Henzler Wildman, K. A.; Lee, D.-K.; Ramamoorthy, A. Determination of α-Helix and β-Sheet Stability in the Solid State: A Solid-state NMR Investigation of Poly(L-alanine). Biopolymers 2002, 64 (5), 246–254. DOI: 10.1002/bip.10180
(34) Sadat, A.; Joye, I. J. Peak Fitting Applied to Fourier Transform Infrared and Raman Spectroscopic Analysis of Proteins. Appl. Sci. 2020, 10 (17), 5918. DOI: 10.3390/app10175918
(35) Goormaghtigh, E.; Ruysschaert, J.-M.; Raussens, V. Evaluation of the Information Content in Infrared Spectra for Protein Secondary Structure Determination. Biophys. J. 2006, 90 (8), 2946–2957. DOI: 10.1529/biophysj.105.072017
(36) Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I. S.; Przekora, A. Spectroscopic Studies on the Temperature-Dependent Molecular Arrangements in Hybrid Chitosan/1,3-β-D-Glucan Polymeric Matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. DOI: 10.1016/j.ijbiomac.2020.05.155
(37) Curley, D. M.; Kumosinski, T. F.; Unruh, J. J.; Farrell, H. M. Changes in the Secondary Structure of Bovine Casein by Fourier Transform Infrared Spectroscopy: Effects of Calcium and Temperature. J. Dairy Sci. 1998, 81 (12), 3154–3162. DOI: 10.3168/jds.S0022-0302(98)75881-3
(38) Saeid, A.; Chojnacka, K. Sulfuric Acid. In Encyclopedia of Toxicology (Third Edition), Wexler, P. Ed.; Academic Press, 2014; pp 424–426.
(39) Vyskočilová, G.; Ebersbach, M.; Kopecká, R.; Prokeš, L.; Příhoda, J. Model Study of the Leather Degradation by Oxidation and Hydrolysis. Heritage Sci. 2019, 7 (1), 26. DOI: 10.1186/s40494-019-0269-7
(40) Badea, E.; Miu, L.; Budrugeac, P.; Giurginca, M.; Mašić, A.; Badea, N.; Della Gatta, G. Study of Deterioration of Historical Parchments by Various Thermal Analysis Techniques Complemented by SEM, FTIR, UV-Vis-NIR and Unilateral NMR Investigations. J. Therm. Anal. Calorim. 2008, 91 (1), 17–27. DOI: 10.1007/s10973-007-8513-x
Alireza Koochakzaei is with the Faculty of Cultural Materials Conservation at Tabriz Islamic Art University, in Tabriz, Iran. Direct correspondence to: a.koochakzaei@tabriziau.ac.ir or alireza.k.1989@gmail.com ●

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.