Development of a High-Throughput LC–MS-MS Assay for 13 Commonly Prescribed Pain Management Drugs from Urine with Cleanup Using Solid-Phase Extraction
Special Issues
Fast turnaround time is critical in the clinical testing environment. Here, fast liquid chromatography (LC) technologies were utilized for the comprehensive assay of commonly prescribed pain management drugs in under 2 min. The use of fast LC also provided significantly improved sensitivity. A mini-validation for these analytes in human urine was performed and acceptable values for accuracy, precision, linearity, lot-to-lot variability, and matrix effects were demonstrated for each analyte.
Chronic pain affects approximately 86 million people in the United States (1). Pain management centers have been established all over the country to help treat these chronic conditions. During treatment, routine testing is critical to monitor compliance and prevent abuse. Because the average age of healthcare patients continues to increase, the demand for pain management is growing rapidly. To keep up with demand, less expensive, faster, more accurate, and reproducible methods for analyzing the presence and concentration of pain management medications in vivo are needed.
Urine is commonly used for pain management testing as it is a copiously available bodily fluid that can be collected easily. The constituents in urine and their concentration vary significantly between patients and are dependent upon diet, hydration, and health condition. Variations in the endogenous components in urine can impact quantitation in methods using liquid chromatography with mass spectrometric detection (LC–MS-MS). Coelution of endogenous urine components with the analytes of interest can cause matrix effects that result in enhancement or suppression of the MS signal, which impacts the quantitative accuracy of the assay. Removal of endogenous components using solid-phase extraction (SPE) is an effective method for reducing potential matrix interferences before LC–MS-MS analysis.
Typically SPE and gas chromatography (GC)–MS have been used to perform the analysis of opioids in urine. Most GC–MS methods require 7 min or longer from injection to injection. The method described here couples sample cleanup and concentration using strong-cation-exchange SPE with rapid LC–MS-MS analysis (<2 min) for 13 commonly prescribed pain management compounds in human urine.
Methods
A bioanalytical validation for a new SPE and LC–MS-MS procedure for the analysis of pain management drugs in human urine was performed. Five different lots of urine samples were spiked at therapeutically relevant concentrations with 13 commonly prescribed drugs, including hydrocodone, naltrexone, tramadol, and fentanyl. All drug standards were obtained from Cerilliant Corporation (Round Rock, Texas). The lower limit of quantitation (LLOQ) was targeted at 10× below the therapeutic level with less than 20% RSD at LLOQ. The upper limit of quantitation (ULOQ) was set at 10× above the therapeutic cutoff.
Urine samples were first hydrolyzed to convert the glucuronide metabolites to native form using β-glucuronidase. This was done by first weighing 1 g of β-glucuronidase and diluting with 20 mL of deionized water. A 100-µL aliquot of this solution was added to 1 mL of urine and vortexed to mix (6200 units in 1 mL of urine). Samples were incubated for 2 h at 60 °C and then were centrifuged for 10–15 min at 9500–13,000 rpm to form a pellet of protein and solids. The clarified supernatant (~1 mL) was then removed and diluted with 1 mL of 100 mM ammonium acetate buffer (pH 5.95).
Cleanup was performed using polymer-based strong-cation-exchange media (Strata-X-C 30 mg/3 mL, Phenomenex, Torrance, California) to remove endogenous matrix components. Analysis was performed using a high performance liquid chromatography (HPLC) system (1200SL, Agilent, Santa Clara, California) coupled to a 4000 QTRAP MS system with a Turbo V ion source (Applied Biosystems, Foster City, California). The chromatographic column used was a 50 mm × 2.1 mm, 2.6-µm C18 (Kinetex, Phenomenex, Torrance, California), which is based upon new core-shell particle technology.
SPE Protocol
Cartridge: Strata-X-C 30 mg/3 mL
Condition: 1 mL methanol
Equilibrate: 1 mL 100 mM ammonium acetate buffer (pH 5.95)
Load: Pretreated sample diluted with 1 mL 100 mM ammonium acetate buffer (pH 5.95)
Wash: 2 × 1 mL 100 mM ammonium acetate buffer (pH 5.95)
Wash: 2 × 1 mL methanol
Dry: Full vacuum for 2–5 min to remove residual water and methanol
Elute: 2 × 0.5 mL 60:24:16 ethyl acetate–isopropanol–ammonium hydroxide
Evaporate to dryness using nitrogen gas and a heating block at 45 °C, then reconstitute in 1 mL of initial mobile phase for LC–MS-MS analysis.
Results and Discussion
The analysis of all 13 pain management drugs was completed in less than 1.5 min with a total gradient cycle time from injection to injection of less than 2.2 min (Figure 1). The use of the core-shell HPLC column provided extremely narrow chromatographic bands with average peak widths at base of ~1 s. These extremely narrow peak widths challenged the scan rate of the MS system operated in triple-quadrupole mode and required the use of scheduled MRM transitions to ensure enough scans per peak to maintain chromatographic resolution. The narrow peak widths also translated into significant increases in MS sensitivity, requiring the dilution of some samples by 20-fold to bring concentrations within the dynamic range of the system.

Figure 1
The columns used provided efficiencies similar to sub-2 µm particles at back pressures that allow them to be used on conventional HPLC systems. Figure 2 shows the core-shell particle geometry. These particles are manufactured by growing a 0.35-µm porous layer on a 1.9-µm nonporous core. The result is 2.6-µm particles..
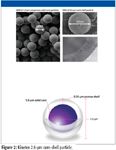
Figure 2
Table I summarizes the results obtained using the procedure described. The chromatographic flow rate of 1.2 mL/min enabled the fast separation of the 13 compounds and the internal standards, with retention times ranging from 0.22 min (2 #3215; void, 0.075 min) for morphine to 1.2 min for methadone. Some internal standards were used as reference for analytes where the deuterated analog was not readily available. The analytical method gave an LLOQ of 2.5 ng/mL with accuracy of 83–98% for all compounds with the exception of fentanyl, which had an LLOQ of 0.05 ng/mL due to its low therapeutic concentration. At LLOQ and 3 × LLOQ, most RSDs were well below 15%, indicating a wide linear working range for all compounds. Method performance at cutoff was better than at the 3 × LLOQ, indicating a possible dilution error during sample preparation. Future work will be done to confirm this. The cutoff concentrations are defined as 300 ppb for all compounds except for fentanyl. Due to the relatively high pharmacological efficacy of fentanyl, ~50 times greater than morphine, the linear analytical range of fentanyl was 50× lower than the other opioids. At cutoff concentrations, all compounds were analyzed with 84–102% accuracy and 5% or less RSD values. Matrix effects were evaluated by determining lot-to-lot precision and accuracy using five different urine donors. At the cutoff concentration the RSD values were less than 7% with high accuracy across all donors. Figure 3 shows an example calibration graph spanning the linear range of 2.5–2500 ppb for morphine, with 1/x2 weighting; r = 0.9912.

Table I: LCâMS-MS results from bioanalytical validation protocol
The high selectivity of LC–MS-MS greatly reduces interferences that affect analyte quantitation. This high selectivity can reduce sample cleanup requirements; however, reduced sample cleanup allows more endogenous material to accumulate in the MS source, which ultimately can increase the frequency of maintenance. The use of SPE for sample clean-up greatly reduces matrix interferences while also significantly reducing the contaminant load on the MS source. The effect of SPE was most dramatic for fentanyl due to its 5 ng/mL cutoff concentration. Using a 30-mg bed mass significantly reduces the solvent volume required for the conditioning, wash, and elution steps. This reduced solvent volume translated into significant cost and time savings for the subsequent blowdown steps.

Figure 3
The SPE protocol utilized has been optimized for the 13 analytes and internal standards. The basic pKa values of the cationic analytes are all close to 8. The buffer chosen, ammonium acetate, is adjusted to pH 5.95 in order to protonate and charge the cationic analytes at 2 pH units below their pKa values. This pH ensures the target analytes are fully protonated maximizing retention and recovery with the sulfonic acid ligand on the SPE medium. Use of ion-exchange retention allows 100% methanol wash to more effectively remove endogenous contaminants including those with lower basic pKa values, such as urobilin, which, when present, may appear yellow. The selectivity of the elution solvent composition, 60:24:16 ethyl acetate–isopropanol–ammonium hydroxide, was optimized to allow elution of the analytes of interest while leaving any remaining endogenous components on the sorbent. Optimized wash and elution solutions yielded clearer and cleaner extracts (Figure 4) and more reproducible and accurate chromatography. The sorbent mass was also optimized to 30 mg of media in a 3 mL tube. The high ion-exchange capacity of the media allows processing small sample volumes even at low concentrations. Due to the high sensitivity of the method, only 1 mL of urine was required for SPE. Using less sorbent reduces cost and solvent consumption and reduces elution volumes, thereby saving time during evaporation, an important consideration for high sample throughput and increased productivity.

Figure 4
Ultrahigh efficiency and fast chromatographic separations result in very narrow peak widths, enhancing resolution and sensitivity. However, these very narrow peaks also present detection challenges when using MS. This is especially true for this method, in which two MRM transitions must be monitored concurrently for each of the thirteen compounds and one for each of the eight internal standards. To optimize the acquisition rate on the MS system, a scheduled MRM window of 6 s was used to get ~20 data points per second (15–18 Hz experimentally). Sufficient sampling of the chromatographic peak is important to maintain resolution between closely eluted bands including other analytes as well as possible interferences. The MRM settings used here are highly recommended because the separation provided by the column produces very narrow, low-volume peaks.
Additionally, when using the columns described here, LC–MS-MS instrumentation should be optimized so the narrow analyte bands are not compromised by extracolumn band broadening. The instrumentation used for this experiment was optimized by bypassing the MS grounding union, eliminating the diverter valve, and utilizing a near-zero dead volume in-line column filter (KrudKatcher Ultra, Phenomenex) to protect the column. It is recommended that the narrowest internal diameter and shortest lengths of connecting tubing possible be used. The effects of dispersion also can be minimized by using high flow rates, injecting the sample in weaker solvent than the initial mobile phase, and running steep gradients when possible.
Conclusion
A bioanalytical method validation was performed for 13 commonly prescribed pain-management drugs in human urine. Accuracy, precision, and lot-to-lot matrix effects were all within expectations for bioanalytical mthods. The use of the polymeric SPE discussed here greatly reduces matrix interferences while also providing clean sample extracts. The 30 mg/3 mL format reduced the amount of solvent used for processing and the time required for blowdown steps. The columns used provided fast run times and improved method sensitivity. This prevalidated solution greatly reduces the time required for implementation in any laboratory interested in analyzing pain management samples.
13 pain management opioids were resolved in 1.5 min with 1-s wide peaks at base on a 50 mm × 2.1 mm, 2.6-µm dp C18 column. Sensitivity, reproducibility, and productivity are dramatically improved after efficient sample cleanup and concentration using the SPE described here. The combined SPE LC–MS-MS analysis provides significant reductions in analysis time, labor requirements, and cost per sample while increasing sensitivity, accuracy, and precision.
A. Carl Sanchez, Philip J. Koerner, and Sky Countryman are with Phenomenex, Inc., Torrance, California.
References
(1) American Chronic Pain Association website.

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.