Nontargeted Screening and Accurate Mass Confirmation of Pesticides Using High-Resolution LC–Orbital Trap Mass Spectrometry
Special Issues
The increasing use of pesticide testing coupled with reductions in maximum permissible residue levels of pesticides in food have driven demand for fast, sensitive, and cost-effective analytical methods for high-throughput screening of multiclass pesticides in food. Detection of 510 pesticides at low parts-per-billion levels can be achieved within minutes using orbital trap technology. The high resolving power of these systems enables accurate mass confirmation of all compounds, including isobaric pesticides. This article will provide an overview of current legislation and illustrate how mass spectrometry instrumentation can enable fast and accurate pesticide screening.
Growing food safety concerns and the globalization of agricultural trade have triggered the enforcement of stringent pesticide regulations worldwide. These different national regulations result in considerable diversity in the level of food protection on a global basis. For example, in 2007, the U.S. Environmental Protection Agency (EPA) completed a 10-year reassessment of 9721 pesticide tolerances to meet more stringent safety standards and recommended the revocation or modification of thousands of uses of pesticides in food (1). China published national standard GB 2763-2005 in 2005, which established 478 maximum residue levels (MRLs) for 136 pesticides (2). Japan's Positive List System, introduced in 2006, established MRLs for hundreds of agricultural chemicals, including approximately 400 pesticides in food, and set a uniform limit of 10 ppb to chemicals for which MRLs have not been determined (3). These regulations have fueled the need for faster, more sensitive and cost-effective analytical methods for high-throughput screening of multiclass pesticide residues in food.
Pesticides in food traditionally have been monitored and quantified using gas chromatography (GC) coupled with either selective detectors (for example, electron capture) or mass spectrometry (MS). Gas chromatography (GC)–MS continues to be widely used in pesticide analysis because it is highly selective, provides confirmation of multiple classes of pesticides in a single analytical run, and is relatively inexpensive and easy to operate. However, GC–MS cannot detect polar, thermally unstable, or low volatility compounds without derivatization.
Recent improvements in liquid chromatography (LC) throughput and MS detection capabilities have led to a surge in the use of LC–MS-based techniques for screening, confirmation, and quantitation of ultratrace levels of multiclass pesticide residues, including those that are not GC-amenable. LC–triple-quadrupole tandem MS (LC–MS-MS) enables highly selective and sensitive quantitation and confirmation of hundreds of target pesticides in a single run, but this approach requires extensive compound-dependent parameter optimization and cannot be used to screen for untargeted pesticides.
Full-scan approaches using high performance time-of-flight (TOF) or single-stage orbital trap mass spectrometers coupled to ultrahigh pressure LC (UHPLC) facilitate rapid and sensitive screening and detection of LC-amenable pesticide residues present in a sample. The superior resolving power of the single-stage orbital trap mass spectrometer up to 100,000 FWHM compared to that of TOF instruments (10,000–20,000 FWHM) ensures the high mass accuracy required for complex sample analysis (4). Full-scan UHPLC–single-stage orbital trap MS can be used to screen a virtually limitless number of pesticides and, unlike MS-MS methods, does not require compound-dependent parameter optimization. High-resolution LC–MS instrumentation, however, can be cost-prohibitive for many routine monitoring laboratories.
Newly designed LC–MS single-stage orbital trap mass spectrometers facilitate reliable mass measurements of complex samples and enable confident discrimination of coeluted isobaric compounds in complex samples at a lower cost (5,6). In addition, a wide in-scan dynamic range of 3–4 orders of magnitude facilitates the detection of trace levels of compounds in the presence of highly abundant matrix interferences. High scan speeds and polarity switching ensure full compatibility with both UHPLC and high-throughput methods. Cost-effective and easy to operate, these systems are an ideal tool for compliance monitoring in regulatory laboratories.
Goal
This study demonstrates rapid screening and accurate mass confirmation of 510 pesticides at low ppb levels using a UHPLC system (Accela, Thermo Fisher Scientific, Madison, Wisconsin) coupled to a high-resolution benchtop single-stage Orbitrap mass spectrometer (Exactive, Thermo Fisher Scientific) and an autosampler (PAL, CTC Analytics, Carrboro, North Carolina). Detection of the 510 pesticides at low parts-per-billion levels was achieved within 12 min. The high resolving power of the single-stage Orbitrap platform enabled precise mass confirmation of all compounds, including isobaric pesticides. The system is ideally suited for routine, comprehensive screening of targeted and nontargeted pesticides at or below the 0.01 mg/kg (10 ppb) default limit set by EU and Japanese legislation.
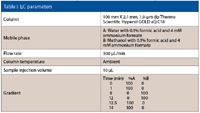
Table I: LC parameters
Experimental
Pesticide standards were obtained from the U.S. Food and Drug Administration (FDA). A stock solution of a mixture of 510 pesticides was prepared at a concentration of 3 mg/L. Calibration solutions with concentrations of 1–250 ppb were prepared by serial dilution of the stock solution in 50:50 (v/v) acetonitrile–water. Spiked spinach samples were prepared for analysis using a modified QuEChERS (quick, easy, cheap, effective, rugged and safe) sample preparation procedure (6). Malathion-d6 was used as an internal standard for calibration.
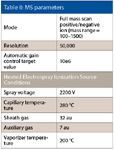
Table II: MS parameters
The LC and MS experimental parameters are detailed in Table I and Table II. Data acquisition was performed using proprietary software (Xcalibur, Thermo Fisher Scientific) as was data processing (Pathfinder, Thermo Fisher Scientific).
Results and Discussion
Experimental results demonstrate that UHPLC improves chromatographic resolution, speed, and sensitivity, and when coupled to MS, it facilitates rapid, high-throughput analysis of challenging samples. Using UHPLC single-stage orbital trap MS, a mixture of 510 pesticides representing a broad spectrum of chemical classes was separated and detected within 12 min. High resolution (50,000) and high mass accuracy (< 5 ppm without internal calibration for most compounds) enabled identification of all analytes. Separation of isobaric pesticides was achieved only at the high resolving powers provided by single-stage orbital trap MS, as demonstrated in Figure 1.
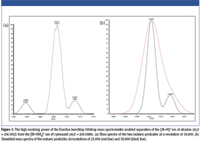
Figure 1
Excellent linearity in detector response was observed over the range of 1–250 ppb, with correlation coefficients greater than 0.99 for the majority of pesticides. For the concentration range studied (1–250 ppb), limits of quantitation (LOQs) were estimated from triplicate injections (CV < 15%) of standard solutions at concentration levels corresponding to a signal-to-noise ratio of 10. LOQs ranged from 1–50 ppb and for 499 pesticides LOQs were at or below 10 ppb, which is the MRL imposed by EU and Japanese regulations.
To evaluate the applicability of the technique for complex food samples, UHPLC–single-stage orbital trap MS was used to screen for pesticides extracted from a spiked spinach matrix. An extraction procedure based on fast and efficient QuEChERS methodology was used to facilitate rapid high-throughput multiresidue analysis. Extracted ion chromatograms and calibration curves for six pesticides extracted from the spiked spinach matrix are depicted in Figure 2.
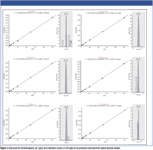
Figure 2
The detection and quantitation capabilities of the method were assessed using the EPA method detection limit (MDL) procedure (7). For all pesticides, limits of detection (LODs) and LOQs were lower than 1 ppb.
Conclusion
Strict worldwide pesticide regulations have created an increasing need for rapid, sensitive, reliable and cost-effective analytical techniques capable of facilitating high-throughput screening of multiclass pesticides in food. In response, UHPLC–single-stage orbital trap MS has emerged as a viable method for fast multiresidue screening of pesticides at low parts-per-billion levels. The high mass resolution and accuracy of the technique enable dependable identification of all compounds at or below the default limits set by legislation. The technique is ideally suited for the routine monitoring of targeted and nontargeted pesticides by regulatory laboratories.
Allen Zhang, James S. Chang, Christine Gu, and Mark Sanders are with Thermo Scientific, Madison, Wisconsin.
References
(1) Environmental Protection Agency, Accomplishments under the Food Quality Protection Act (FQPA), http://www.epa.gov/pesticides/regulating/laws/fqpa/fqpa_accomplishments.htm
(2) National Standard GB 2763-2005, "Maximum residue limits for pesticides in food", Standardization Administration of the People's Republic of China, 2005.
(3) Japanese Ministry of Health, Labor and Welfare, Department of Food Safety, Introduction of the Positive List System for Agricultural Chemical Residues in Foods, http://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228/introduction.html
(4) "High Resolution and Precise Mass Accuracy: A Perfect Combination for Food and Feed Analysis in Complex Matrices," Thermo Scientific Application Note 30163, 2008.
(5) "The Thermo Scientific Exactive Benchtop LC/MS Orbitrap Mass Spectrometer," Thermo Scientific Application Note 30162, 2008.
(6) AOAC Official Method 2007.01. "Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. Gas Chromatography/Mass Spectrometry and Liquid Chromatography/Tandem Mass Spectrometry."

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.