Dosing Vehicle Removal in Discovery Phase Pharmacokinetic Studies Using High-Field Asymmetric Waveform Ion Mobility Spectrometry
Special Issues
This article describes the ability to increase the sensitivity for a target compound in the presence of high-level background impurities by removing the dosing vehicle using a high-field asymmetric waveform ion mobility spectrometry gas-phase separation before mass spectrometry analysis.
This article describes the ability to increase the sensitivity for a target compound in the presence of high-level background impurities by removing the dosing vehicle using a high-field asymmetric waveform ion mobility spectrometry gas-phase separation before mass spectrometry analysis.
Identification and quantification of in vitro and in vivo metabolic products is a routine analytical procedure in the pharmaceutical industry. The presence of low levels of toxic metabolites must be determined in the drug discovery process to avoid costly development of ill-fated lead candidates. High-throughput screening of pharmacokinetic products of larger libraries of samples in early phase discovery is becoming standard; combining these two assays helps to increase throughput further and reduce costs.
Various methods are currently employed to carry out these studies, many of which include liquid chromatography (LC) separation followed by numerous mass spectrometry (MS) analyses. Recently, the chromatographic time scale has been compressed with the development of ultrahigh-pressure liquid chromatography (UHPLC), utilizing smaller diameter (for example, 1.9 μm) particle columns operating at pressures in excess of 5000 psi, which allows for higher throughput. Most recently, high-field asymmetric waveform ion mobility spectrometry (FAIMS) has been used for rapid analysis of samples with little or no cleanup, and with or without chromatographic separation (1).
FAIMS Separation Technology
The FAIMS process separates gas-phase, dry ions at atmospheric pressure. Desolvated ions enter the annular region between two concentric cylindrical FAIMS electrodes and are transported toward the mass spectrometer entrance via a flow of clean, dry gas. Within the gas-filled region of the electrodes, the ions oscillate in alternating high and low electric fields. The waveform is asymmetric; the high field is applied for one time unit followed by an opposite-polarity low field component applied for a different unit of time. Separation of ions during the two opposite polarity phases of the waveform is caused by mobility differences of the ions under the high- and low-field conditions (1).
Over time, the difference in mobility causes ions to travel toward one or the other electrode. A low dc voltage compensates for this migration, resulting in a select ion subset being transmitted into the mass spectrometer. The magnitude of the so-called compensation voltage (CV) is compound-dependent. Ions requiring a different CV for transmission are removed from the ion beam; thus, selectivity is achieved. The CV can be set to a constant value or stepped during an LC–MS run to coincide with the elution of specific analytes. High analyte transmission efficiency is achieved with this increase in selectivity by means of a unique ion-focusing mechanism arising from the cylindrical geometry of the FAIMS electrodes.
FAIMS separation is depicted in Figure 1 (2). An asymmetric RF waveform comprising both a high-field (red) and a low-field (blue) component is applied to one FAIMS electrode. During the high-field pulse, the ions move with mobility μH along the axis perpendicular to the ion axis; during the low-field pulse, the ions move with mobility μL. The difference in mobilities results in a net motion toward one of the electrodes. This net motion is compensated for by the CV.
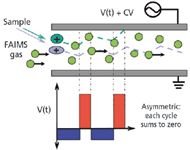
Figure 1: Diagram of use of FAIMS for separation.
Ion mobility is related to shape, charge, size, and other mass-independent properties of molecules, so FAIMS can be used to separate ions of the same nominal mass-to-charge ratio (m/z) but with differing structures. There are numerous advantages to FAIMS in chemical analysis. LC–MS-MS is a highly selective technique for bioanalysis, but for challenging assays it often is not selective enough. Issues such as high chemical background, coeluting isobaric ions, and long analytical run times force researchers to redevelop and validate assays. This process is time-consuming and costly, with no guarantee of success. FAIMS is a unique problem-solving tool for LC–MS methods development, enabling many of the aforementioned challenges to be solved quickly and cost effectively (3).
The FAIMS device is installed in the atmospheric pressure region between the ion source and the mass spectrometer. In just a few minutes, the system is ready to exploit three complementary dimensions of separation: LC, FAIMS, and MS. The simplicity of the hardware design, the ease of installation and use, and the speed at which challenges are solved make FAIMS a technique that should be used early in the LC–MS method development process. The increased selectivity will lead to accelerated method development and improved robustness in LC–MS analyses. FAIMS transmits only dry ions, so solvent clusters are removed almost completely from full-scan mass spectra. Chemical interferences of dissimilar charge but like m/z, such as peptides and small molecules, can be separated. Finally, molecules of like charge and mass, such as a drug and dosing vehicle or endogenous interferences, can be separated (4).
Reducing Chemical Noise in Pharma Studies
It is common practice in pharmaceutical development to conduct discovery-phase pharmacokinetic (PK) studies. In these studies, dosing vehicles such as Tween 80, PEG 400, and methylcellulose are used in high concentrations to dissolve the test compounds in dose formulations. Subsequent qualitative study and quantification of the test compounds must be carried out in the presence of the dosing vehicles, which can cause significant ion suppression and complicate full-scan mass spectra. Conventional LC can be used to separate the dosing vehicle; however, this approach is generally less than 100% efficient, and LC–MS analyses still show the significant presence of polymers.
The effect of ion suppression combined with complicated full-scan mass spectral data makes successful LC–MS analysis of targets difficult or impossible. Reducing or removing multiple chemical interferences can improve spectral quality dramatically and enhance full-scan signal-to-noise ratios (S/N) for the analyte of interest. In this article, the successful atmospheric pressure separation by FAIMS of a large excess of dosing vehicle (PEG) from a test compound (loperamide) before MS analysis is reported.
Methods
HPLC: Not used
Separation: Gas phase differential ion mobility: FAIMS
Gas flow rate: 1.5 L/min
Gas composition: 50% He, 50% N2
CV range: –40 to 0 V
CV scan time: 1.2 min
Sample introduction: Infusion at 2 μL/min
Mobile phase: 50:50 acetonitrile–water with 0.1% formic acid
Mass spectrometer: Linear ion trap
Ionization: Positive nanospray
Spray voltage: 4000 V
Capillary temperature: 200 °C
Results
Full-scan positive ion nanospray ionization (NSI) mode mass spectra were acquired over the range from m/z 300 to m/z 1000 using an LTQ XL linear ion trap mass spectrometer (Thermo Fisher Scientific, San Jose, California) equipped with FAIMS. The CV was scanned from –30 V to 0 V at an approximate rate of 30 V/min. A full-scan mass spectrum obtained using a standard Ion MAX source (no FAIMS separation) is shown on the left-hand side of Figure 2. Without FAIMS separation, evidence of PEG is found over the entire mass range of interest, including at the nominal mass of the target drug, lo-peramide. The mass range between 470 and 488 is expanded on the right-hand side of Figure 2, which clearly shows that the 13 C isotope of the PEG at m/z 476.42 coincides with the mono-isotopic peak for loperamide at m/z 477.42. The typical PEG spectrum is characterized by a series of m/z species, which are separated by 44 mass units across the mass range from 400 to 1000 in Figure 2.

Figure 2: Mass spectrum without FAIMS separation, with mass range between 470 and 488 expanded on the right.
The presence of the PEG dosing vehicle in the sample makes isolation, clean fragmentation, and hence, identification of the target drug difficult, particularly when the target analyte is at low concentrations. Contrast the results in Figure 2 with FAIMS results shown in Figure 3, where the CV is scanned and the PEG and loperamide are clearly separated. In the top panel of Figure 3 is an extracted ion trace for a 1.5 amu window centered at the nominal mass of singly charged, protonated loperamide (m/z 477). The presence of two peaks in the trace indicates several possibilities, including the existence of two conformations of the same molecule or, as in this case, the presence of two molecules of the same nominal mass but with different structures.

Figure 3: Results using FAIMS.
In the middle left panel of Figure 3 is the full-scan mass spectrum obtained at a CV centered on the blue peak, –21 V; in the middle right panel of Figure 3 is the full mass spectrum obtained at a CV centered on the red peak, –12 V. The full-scan mass spectrum obtained at a CV of –21 V contains multiple contributions from the PEG dosing vehicle, including the component at m/z 476.42; the full-scan mass spectrum obtained at a CV of –12 V contains a single component, loperamide, at m/z 477.34.
The full-scan mass spectral S/N has been improved (Figure 2) by greater than an order of magnitude. Verification of the identity of each component is achieved by MS-MS analysis, the results of which are shown for the PEG in the bottom left panel of Figure 3 and for loperamide in the bottom right panel of Figure 3.
Conclusion
FAIMS in combination with the linear ion trap mass spectrometer made possible the detection and identification of the target compound loperamide in the presence of a vast excess of the dosing vehicle PEG 600. The FAIMS full-scan mass spectrum S/N and sensitivity for the target compound were increased significantly. The ability of FAIMS to provide an orthogonal separation based upon shape, size, and charge can be used to reduce dramatically chemical noise due to dosing vehicles.
Julie Horner and Julian Phillips are with Thermo Fisher Scientific, San Jose, California.
References
(1) A.A. Shvartsburg, K. Tang, and R.D. Smith, J. Am. Soc. Mass Spectrom. 16, 2–12 (2005).
(2) A.A. Shvartsburg, K. Tang, and R.D. Smith, Anal. Chem. 76, 7366–7374 (2004).
(3) D.A. Barnett, B. Ells, R. Guevremont, and R.W. Purves, J. Am. Soc. Mass Spectrom. 10, 1279–1284 (1999).
(4) J.T. Kapron, M. Jemal, G. Duncan, B. Kolakowski, and R. Purves, Rapid Commun. Mass Spectrom. 19, 1979–1983 (2005).

Advanced Raman Spectroscopy Method Boosts Precision in Drug Component Detection
April 7th 2025Researchers in China have developed a rapid, non-destructive Raman spectroscopy method that accurately detects active components in complex drug formulations by combining advanced algorithms to eliminate noise and fluorescence interference.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.