Gas Chromatography–Time-of-Flight Mass Spectrometry Utilized As an Effective Weapon Against Illegal Drugs of Abuse
This article presents GC–TOFMS methods developed to identify several major drug classes and chemical functionalities.
Illegal drug use worldwide is at an all-time high. There is a crucial need for fast and accurate analysis to positively identify suspected drugs in criminal investigations. Gas chromatography combined with time-of-flight mass spectrometry (GC–TOFMS) can be a valuable tool for drug testing in forensic crime laboratories. Method development and GC–TOFMS experimentation was conducted in cooperation with a local crime laboratory. The laboratory testing presented will illustrate highly efficient methods and data developed for crime labs that assist in the battle against illegal drugs. Many drug classes have chemical properties that present particular analytical challenges, such as poor detector response, chemical lability, or poor chromatographic peak shape. This article presents GC–TOFMS methods developed for several major drug classes and chemical functionalities. The major drugs included in the initial method development are methamphetamine, ecstasy, and heroin. Robust and accurate analytical methods were developed for clandestine laboratory process samples, identification of confiscated drug substances, and trace-level analysis for suspected drug residues. Finally, a rapid screening method was developed for illegal drugs of abuse from several chemical classes using a single analysis.
The capabilities and advantages of gas chromatography–time-of-flight mass spectrometry (GC–TOFMS) are emphasized by an application showing a fast screening method for 12 drugs of various chemical functionalities in 4.9 min. The benefits of utilizing GC–TOFMS for drug identification analysis include the ability to acquire highly sensitive, full mass range spectra at the fast acquisition rates essential for high-throughput analysis. This work demonstrates the ability of GC–TOFMS to increase laboratory productivity and efficiency while providing indisputable positive identifications for illegal drug analysis in criminal investigations.
Experimental
Confiscated Drugs GC–TOFMS Analysis Method Development
Initial methods were developed for three major drugs of abuse: ecstasy, heroin, and methamphetamine. Drug samples were prepared by dissolving the suspected material in methanol and retaining the soluble portion for analysis. Analytical results were generated with a Pegasus HT GC–TOFMS system (LECO Corp., St. Joseph, Michigan). The MS system was equipped with an Agilent 6890 gas chromatograph (Wilmington, Delaware) and Gerstel MPS2 autosampler (Baltimore, Maryland). LECO ChromaTOF software with True Signal Deconvolution was used for all instrument data acquisition and peak identification. A 30 m × 0.25 mm, 0.25-μm film Rxi-5 ms column (Restek Corp., Bellefonte, Pennsylvania) was used to obtain the necessary chromatographic separation. Helium was used as the carrier gas at a constant flow of 2.0 mL/min. Various chromatographic temperature programs were developed for specific drug types. The GC temperature program developed for methamphetamine and ecstasy consisted of an initial oven temperature of 100 °C for 1.0 min followed by a temperature ramp of 15 °C/min to a final temperature of 280 °C held for 2.0 min, with a total run time of 15.0 min. The methamphetamine and ecstasy samples were introduced into the GC system with a 1-μL injection split 200:1 at 250 °C. The MS transfer line temperature was maintained at 280 °C. The GC temperature program developed for heroin analysis consisted of an initial oven temperature of 120 °C for 0.5 min with an initial temperature ramp of 20 °C/min to 200 °C, followed by a secondary temperature ramp of 10 °C/min to a final temperature of 280 °C, with a final hold time of 5 min for a total run time of 17.5 min. The heroin samples were introduced into the GC system with a 1-μL injection split 10:1 at 200 °C. The MS transfer line temperature was set to 280 °C. Uniform mass spectrometer parameters were used for the initial methods. The mass range was set at 45–450 amu with an acquisition rate of 5 spectra/s. The ion source chamber was set to 225 °C, and the detector voltage was set to 1750 V with an electron energy of –70 eV.

Figure 1: The (unique mass) extracted ion chromatograms for (a) ecstasy, (b) heroin, and (c) methamphetamine by the GCâTOFMS methods developed for routine identification of suspected confiscated drugs.
Results and Discussion for Confiscated Drugs GC–TOFMS Analysis
Methods developed for methamphetamine, ecstasy, and heroin were carried out with multiple injections by the GC–TOFMS system. Analytical results show chromatograms with target analytes labeled according to NIST MS library searches for each drug. Figure 1a shows the extracted ion chromatogram for the drug ecstasy (N-methyl-3,4-methylenedioxyamphetamine) using the unique mass fragment mass-to-charge ratio (m/z) 58. The peak for ecstasy is eluted at 405.6 s with a library similarity of 905 out of 999. The extracted ion chromatogram for heroin (diacetylmorphine) is illustrated in Figure 1b using the unique mass fragment of m/z 81. The peak designated as heroin exhibits a library similarity match of 887 out of 999. Figure 1c shows the extracted ion chromatogram for methamphetamine using the unique mass fragment of m/z 58. The peak for methamphetamine was eluted at 229.9 s with a library similarity of 889 out of 999. Both ecstasy and methamphetamine are sympathomimetic amines (basic drugs), which are known to afford poor chromatographic peak shape. Both analytes exhibit quality chromatographic peak performance by the GC–TOFMS analysis. Heroin is known to be chemically labile and prone to loss of an acetyl group, forming the compound monoacetylmorphine. Monoacetylmorphine can be observed in Figure 1b, and was eluted before heroin at 701.6 s. The method developed shows successful identification of heroin with high library match quality. The methods developed for methamphetamine, ecstasy, and heroin are uncomplicated and robust with analysis run times of less than 20 min. They require minimal sample preparation, without the need for time-consuming sample derivatization.

Figure 2: The analytical ion chromatogram (AIC) of the methods developed for the analysis of suspected "meth" process lab samples: (a) the volatile layer of the meth process lab sample; (b) the analysis of the aqueous layer. Positive identification for methamphetamine is observed in both analyses at a retention time of 223.7 s.
Methamphetamine Process Laboratory Analysis Method Development
Law enforcement agencies confiscate multilayer liquid samples, which are used as extraction solvents in the manufacturing process for methamphetamine, from suspected clandestine "meth labs." The samples contain an aqueous and a volatile layer. Methods were developed to analyze these samples by GC–TOFMS. The GC–TOFMS method used for the meth lab process samples consisted of the same column and method parameters as previously developed for the analysis of methamphetamine and ecstasy. The process sample contained two distinct dingy layers. The top volatile layer was filtered and introduced into the GC system directly with a 1-μL injection, split 200:1 at 200 °C. The aqueous layer was prepared for analysis by extracting a 5-mL aliquot with 2 mL of methylene chloride. The methylene chloride was filtered and introduced into the GC system with a 1-μL injection, split 200:1 at 200 °C. The mass spectrometer method parameters remained unchanged from the previous methamphetamine–ecstasy analyses.

Figure 3: The AIC for the trace-level powder residue analysis by GCâTOFMS. The inset illustrates a trace-level peak for methamphetamine that was found utilizing the softwares deconvolution capabilities.
Results and Discussion for Meth Lab Process Samples Analysis
The results for the meth lab process samples show that trace levels of methamphetamine can be detected even in heavy sample matrix. Positive identifications with high library similarity scores were obtained for both the volatile and aqueous layers of the process laboratory sample. Methamphetamine was detected with a library similarity score of 817 out of 999 for the aqueous sample and a library similarity score of 916 out of 999 for the volatile sample. Note that the extraction solvent of choice used by many clandestine laboratories is Coleman fuel, which is a purified high-octane gasoline. The method development and analysis for the meth lab process samples demonstrates the ability of GC–TOFMS to provide a straightforward and robust analysis for harsh samples with minimal interference from sample matrices.
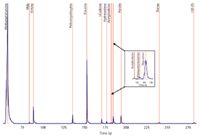
Figure 4: The AIC for the rapid drug screen method of 12 drugs plus acetylcodeine in 4.9 min. The inset shows an expanded view of three closely eluted peaks: acetylcodeine, monoacetylmorphine, and oxycodone.
Trace-Level Powder Residue Analysis Method Development
Trace levels of powder found in proximity to suspected drug users are common sample types encountered by forensic crime laboratories. A GC–TOFMS method was developed on a trace amount of powder in a plastic bag found on a drug abuse suspect. The sample was prepared by dissolving the powder in a 200-μL mixture of methanol and methylene chloride. A 50-μL aliquot was transferred to an autosampler vial for analysis. A 2-μL, splitless injection was made with the injection port temperature maintained at 250 °C. A 30 m × 0.25 mm, 0.25-μm film Rxi-5 ms column (Restek Corp.) was used to obtain the necessary chromatographic separation. Helium was used as the carrier gas at a constant flow of 2.0 mL/min. The GC temperature program developed for the trace-level residue analysis consisted of an initial oven temperature of 100 °C for 1.0 min followed by a temperature ramp of 12 °C/min to a final temperature of 280 °C held for 2.0 min, with a total run time of 18.0 min. The MS system transfer line temperature was set to 280 °C. The mass spectrometer mass range was set at 45–450 amu with an acquisition rate of 5 spectra/s. The ion source chamber was set to 225 °C, and the detector voltage was set to 1750 V with an electron energy of –70 eV.
Results and Discussion for Trace-Level Powder Residue Analysis
The method developed for trace-level residue analysis by GC–TOFMS yielded identification of 40 chemical compounds. Positive identification was made for four drugs of abuse — dextroamphetamine, amphetamine, methamphetamine, and 3,5-dimethoxyamphetamine — with a high degree of certainty. The results show that GC–TOFMS has the capability to identify trace-level residues of illegal substances within contaminating matrices. The inset in Figure 3 shows the unique mass ion chromatogram of m/z 58 for peak 10 with a positive identification for methamphetamine. This example illustrates the ability of TOFMS to acquire nonskewed full-range mass spectral data vital for identification of coeluted or partially coeluted low concentration analytes. The method developed for trace-level residue analysis demonstrated excellent capability to detect and identify illegal drugs, even in heavy sample matrices, using GC–TOFMS.
Rapid Screen Method for 12 Drugs of Abuse Method Development
Crime laboratories have a crucial and immediate need for high-throughput and definitive test methods that will relieve the ongoing sample backlog burden. This GC–TOFMS method was developed to meet the need for fast identification of multiple drug classes in an analysis time of less than 5 min. A mixture of 12 drugs from four chemical classes was prepared as a standard in this rapid drug screen method development. The stock drug mixture was prepared in 5 mL of methanol using high-purity standards and then diluted (10:1) in methanol. A 1-μL injection, split 10:1 at 260 °C, was made. A 20 m × 0.18 mm, 0.18-μm film Rxi-5 ms capillary column (Restek Corp.) was used to obtain the necessary chromatographic separation. Helium was used as the carrier gas at a constant flow of 2.0 mL/min. The GC temperature program was set to an initial oven temperature of 125 °C for 0.10 min, followed by a temperature ramp of 70 °C/min to a final temperature of 300 °C held for 2.3 min, with a total run time of 4.9 min. The MS system transfer line temperature was set to 260 °C. The mass spectrometer mass range was set at 40–450 amu with an acquisition rate of 10 spectra/s. The ion source chamber was set to 200 °C, and the detector voltage was set to 1850 V with an electron energy of –70 eV.
Results and Discussion for Rapid Drug Screen Method
The GC–TOFMS analysis for the rapid drug screen method was able to identify all 12 components of the mixture successfully in 4.9 min. Subsequently, a reproducibility study was conducted by making 10 replicate injections of the 12-component standard. The 10 injections positively identified all target components in the drug mixture. The 12 drugs of abuse plus acetylcodeine were identified with an average library match of 87% in less than 5 min. The analysis method by GC–TOFMS successfully overcame the analytical challenges associated with these drug chemical classes. A simple, robust, and fast method was achieved by GC–TOFMS in 4.9 min, proving the value of this instrumentation for rapid drug screening.
Conclusions
This article presents experimental results demonstrating the capability of GC–TOFMS to provide simple, robust, efficient, and rapid analytical methods for the identification of numerous illegal drugs in a variety of sample types. The results of this work demonstrate that GC–TOFMS is a valuable tool that is highly beneficial for crime laboratories in the fight against illegal drugs of abuse. TOFMS provides fast acquisition and simultaneous sampling of all ions in each mass spectrum, enabling accurate library searches of target analytes even in heavy sample matrices. In areas of serious chromatographic overlap, the automated peak deconvolution provided accurate and reproducible library matches. Robust and accurate analytical methods were developed successfully for identification of drug products in clandestine laboratory process samples, identification of confiscated drug substances, and trace-level analysis for suspected drug residues, including a rapid screening method for 12 illegal drugs of abuse from four different chemical classes in a single 4.9-min analysis. This collaborative effort with a local crime laboratory shows the advantages of GC–TOFMS to increase laboratory throughput while providing definitive identifications for illegal drug criminal investigations. These methods are being applied as an effective analytical tool in the fight against the rising worldwide drug abuse crime problem.
John Heim and Joe Binkley are with LECO Corporation, Life Science and Chemical Analysis Centre, St. Joseph, Michigan.
References
(1) B.A. Vohlken and S.M. Layton, DEA Resources, Microgram Journal, Vol. 1, January–June 2003.
(2) D. Filmore, Today's Chemist, 10(2), 27–32 (2001).
(3) P.D. Andrews, K. Naik, C. Kinemond, and A. ImObersteg, J. Pharm. Practice, 13(3), 226–235 (2000).
(4) D. Blachut, K. Wojtasiewicz, and Z. Czarnocki, Forensic Science Int., 45–62 (2003).
(5) R.F.X. Klein, P.A. Hays, DEA Resources, Microgram Journal, Vol. 1, January–June 2003.

Previewing the American Academy of Forensic Sciences Conference
February 14th 2025This year, the American Academy of Forensic Sciences Conference is taking place from February 17–22, 2025. We highlight the importance of spectroscopy in this field and why we’re covering the conference this year.
Raman Spectroscopy Takes a Leap Forward in Forensic Drug Detection
January 29th 2025Researchers have demonstrated the potential of deep ultraviolet Raman spectroscopy (DUVRS) as a rapid, nondestructive, and sensitive tool for detecting antihistamines like cetirizine in oral fluid samples, paving the way for broader forensic applications.