Exploration of the Use of Raman Microscopy to the Identification of Extractables and Leachables from Polymeric Containers
In a follow-up to my February 2020 column, I started a more systematic study of extractables and leachables. Following a suggestion from Mark Witkowski of the FDA, I looked at three sets of centrifuge vials that were exposed to the following liquids in an effort to evaluate the potential of Raman microscopy to identify compounds exiting in polymers under particular conditions: saline, phosphate buffer, water, saline treatment at 100 0C, phosphate buffer treatment at 100 0C, water treatment at 100 0C, ethanol, chloroform, pH 5, and pH 9. Although all containers were made of polypropylene (PP), they didn’t behave similarly. Compounds that were extracted from PP vials from different manufacturers were not always the same. Although the number of spectral types that are recorded is large, this article focuses on a few whose interpretation is interesting. The goal was to figure out when it makes sense to employ Raman microscopy for such identification. The characteristics considered were ease of sample preparation, the minimum quantity of material amenable to analysis, and the quality of the identification.
The selection of solvents for generating extractables and leachables from these polypropylene vials was informed by two USP publications (1,2). Polished stainless steel slides were used as substrates for the analysis because most metals, including stainless steel, do not have a Raman spectrum. These slides virtually provided a background-free substrate for the spectra. For each vial, samples were prepared immediately after receipt and then approximately 10 days after receipt. After the droplets dried, a mosaic micrograph was recorded with a low magnification objective. Then, the regions of interest were selected and imaged at 50x or 100× magnification before spectra were recorded. Interestingly, it was the 638-nm laser line that provided the best results; there was often fluorescence at 532 nm, the signal-to-noise (S/N) using the 785-nm laser was significantly poorer, and the sensitivity in the CH/NH region was compromised. Because of differences in solubility, if there are more than one species in a sample, they tend to separate during precipitation so spectra of the different species are recorded separately. In all cases, spectra were recorded from multiple spots with similar morphology to ensure that a spectrum was representative of the sample. Although multiple spectra can be recorded with the mapping function (that is, in a two-dimensional [2D] area with evenly spaced points), we more often used a function where we could select points in the image. By selecting spots with the same morphology, the analysis time was significantly reduced compared to what would have been required for a map acquisition. To improve the S/N of the spectra, identical multiple spectra from a given data set were averaged together.
We used KnowItAll (KIA) (3) software (Wiley) for spectral interpretation. To optimize the relative intensities in the spectra for matching, spectra were collected with the instrument correction spectrum (ICS) function turned on. If a suitable match did not appear, we used standard rules for spectral interpretation to provide an identification of at least the class of molecule represented by the spectrum.
Identification of Unknowns
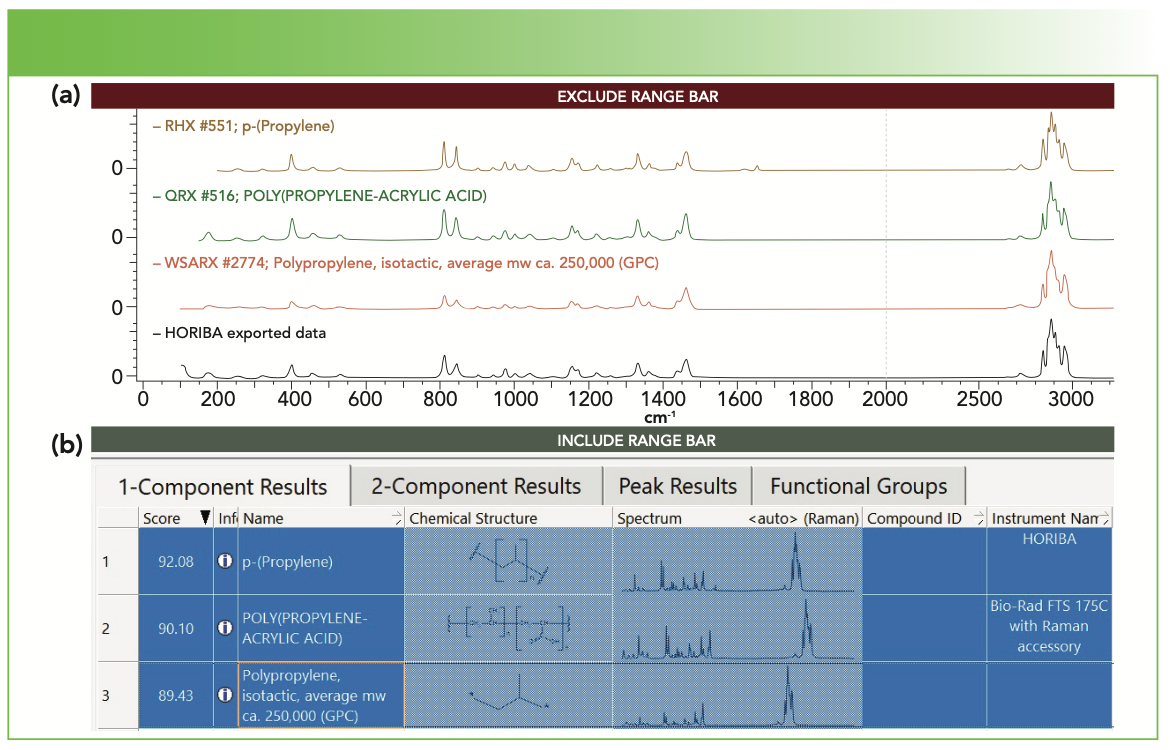
To demonstrate the functionality of our method, Figure 1 shows the spectrum of one of the vials, as identified as polypropylene (PP) in KIA. However, you may notice that I have not chosen the spectrum with the highest hit quality index (HQI). The first bands near 1620 and 1650 cm-1 that are spectrum, with an HQI of 92.1, has extra not in my spectrum. The second spectrum with an HQI of 90.1, is identified as a copolymer of PP and polyacrylic acid (6%). For the moment, I am assuming that these tubes are pure PP, possibly containing some additives, so I have chosen the third entry. The ambiguity in even such a simple query indicates the reasons that some purist spectroscopists warn against solely using spectral data bases for identification.
FIGURE 1: (a) Raman microscope spectrum of a centrifuge vial (bottom black spectrum) with (b) the top three matches in KIA software (see text for discussion). Top matching spectra are shown in both (a) and (b).
Results from Vials Exposed to Saline
Figure 2 shows mosaic images of deposits from centrifuge vials from the three vendors. Many of the deposits indicate the morphology of NaCl crystals, but with material of more disordered morphology adhering to the NaCl cubes. Of course, NaCl has no first-order Raman spectrum, so the spectra that we show were acquired from material clinging to the salt crystals. Figure 3 shows spectra of deposits from vials exposed to saline from each of the 3 suppliers, with the spectrum of PP shown at the bottom for easy reference. The startling observation is that each vial yielded different spectra. When a spectrum indicated the presence of elemental carbon, its two bands had been eliminated manually before plotting in Figure 3 or importing into the KIA software.
FIGURE 2: Low magnification mosaic micrographs of deposits from the saline-exposed centrifuge vials of the three sources studied here. From left to right: first, second, and third vendors.
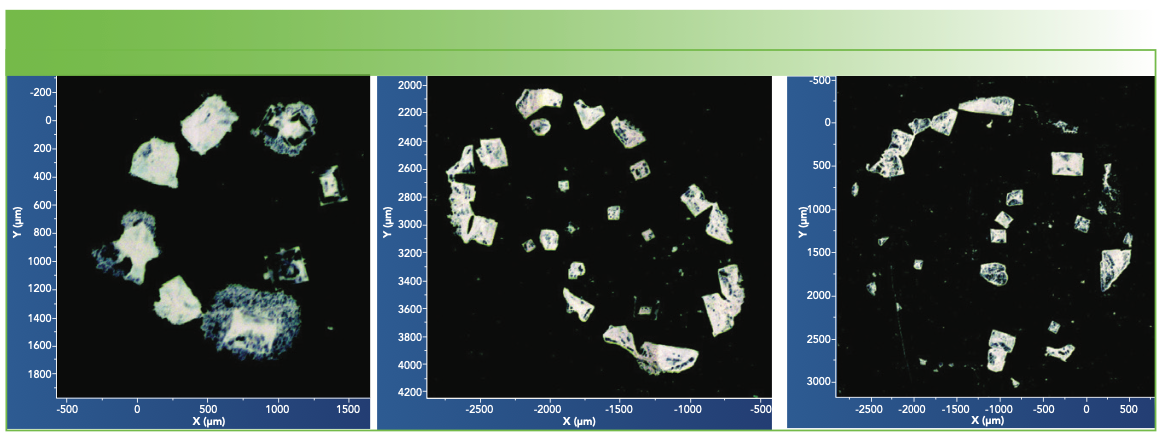
FIGURE 3: Spectra of deposits from the saline solutions in centrifuge vials of the three vendors. Note that two different species were observed in the vial of the second vendor (second and third spectra from the top). The spectrum of one of the polypropylene vials is shown on the bottom as a reference.
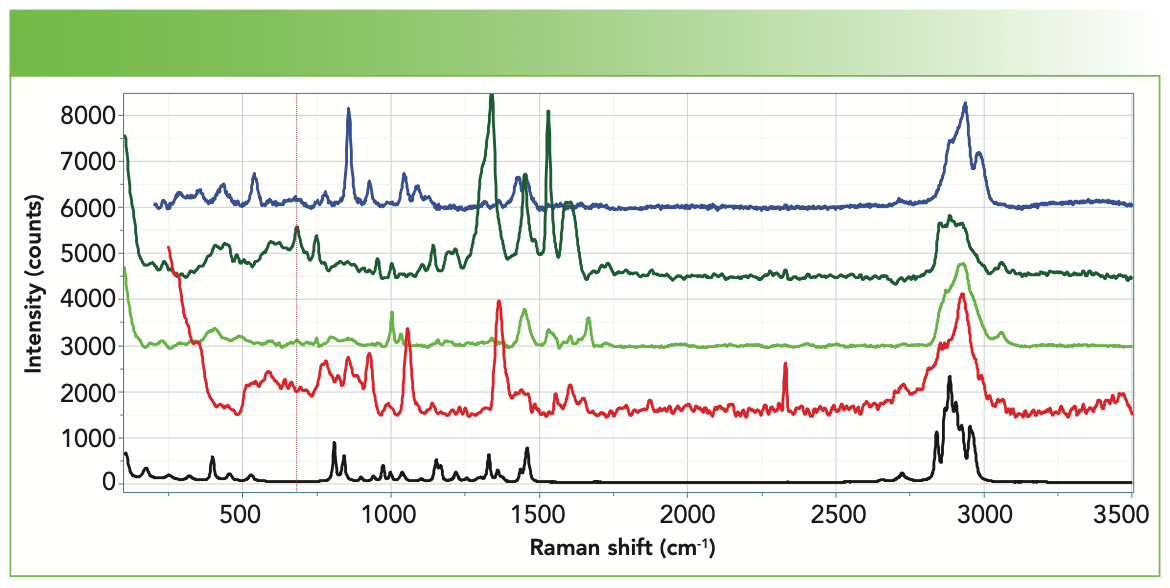
Each spectrum was examined separately in the KIA software, and the results are displayed in Figure 4. None of the matches are as good as that for PP, but there still is ample information present. I should explain how I decide that a match is “good” when a match of the quality of the PP does not appear. The most important thing to understand is that the HQI represents the numerical overlap of the query spectrum with a spectrum from the data base. If there is a baseline, it will tend to overwhelm the HQI without really representing the species present. So the first thing to do is to subtract any baseline in your query spectrum and to enable baseline subtraction in KIA. I have also found that if there are broad bands in my spectrum, for instance from carbon, the searching algorithm finds carbon before anything else. Therefore, it is often helpful to remove the carbon contribution manually from the query spectrum. I will be the first to admit that doing so is not rigorous, but it is effective. That is because looking for a mixture spectrum with sharp bands and the carbon spectrum is not always straightforward because the carbon spectrum is so variable.
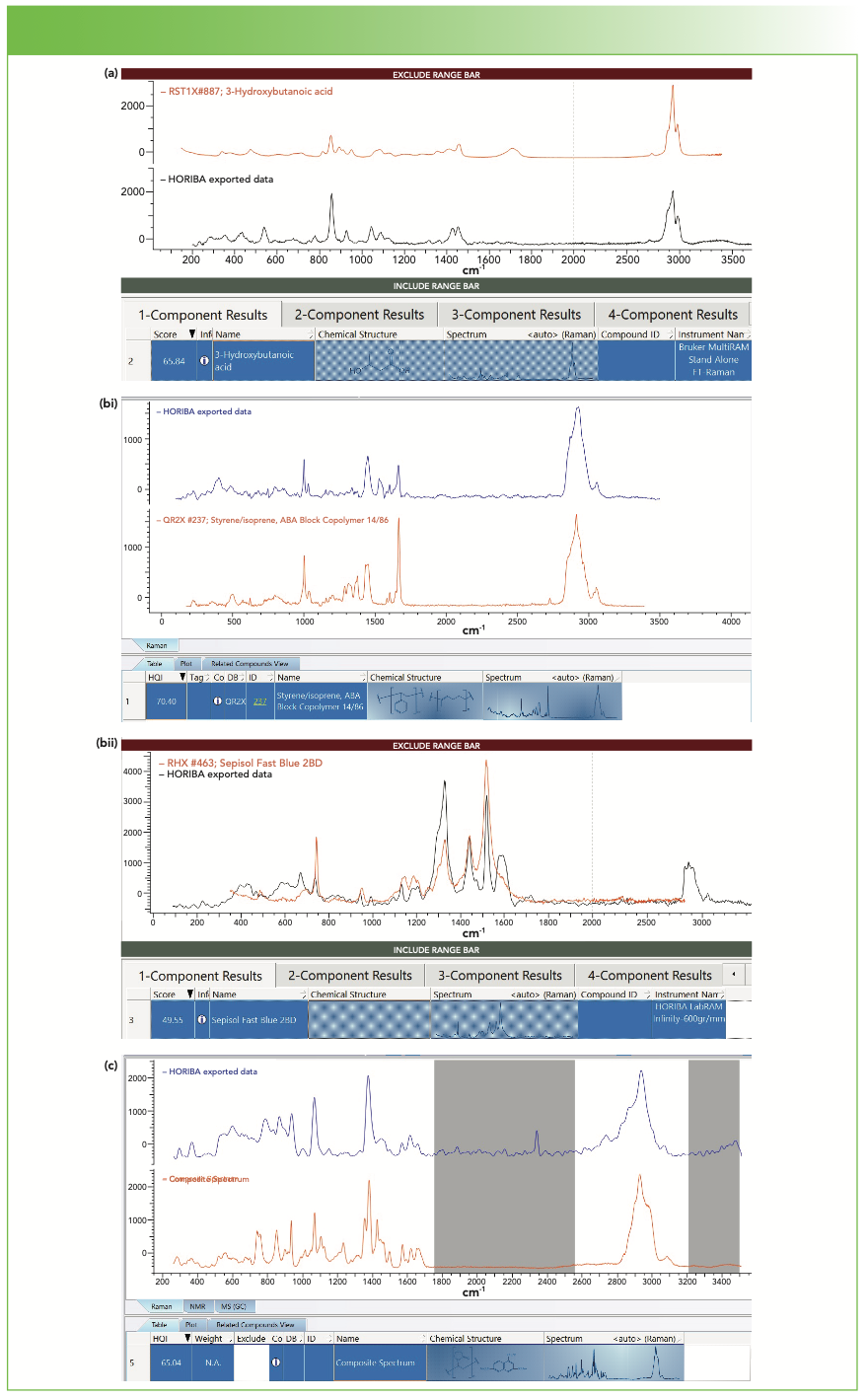
FIGURE 4: (a) Spectrum of extract from the first vial (black spectrum) exposed to saline with the “best” match spectrum being 3-hydroxybutanoic acid, found in IDExpert matching software (red spectrum). (bi) Spectrum of extract from the second vial exposed to saline (red spectrum) with the “best” match identified as a block copolymer styrene/isoprene as displayed in SearchIt (blue spectrum). (bii) Spectrum of a second extract species from the second vial (black spectrum) exposed to saline with the “best” match as displayed in ID Expert, a dye called sepisol fast blue (red spectrum). (c) Spectrum of extract from the vial of the third vendor exposed to saline (blue spectrum) with the “best” match being a mixture of poly (N-vinyl pyrollidone) and naphthalene 1,3,6 trisulfonic acid trisodium salt, as found in SearchIt (red composite spectrum).
So if a really good match does not pop up, the question is how to find the “best” match. What I do is to examine the listing in software, which is arranged in decreasing values for HQI while also examining the spectral pattern. I want to find a hit that has prominent bands in the same region as the prominent bands in the query spectrum. Unfortunately, this is not a simple selection of the spectrum with the highest HQI because of the reason explained in the previous paragraph.
So now let’s consider the results of the species found in the saline solutions from the three vendors’ vials. The first is a small molecule of 3-hydroxy butanoic acid. The second had two spectra—the first, a block copolymer of styrene isoprene, and the second, a spectrum of a dye, Sepisol Fast Blue. The spectrum of the deposit from the third vendor’s vial matched best to a mixture of poly (N-vinyl pyrollidone) and naphthalene 1,3,6 trisulfonic acid trisodium salt.
In Figure 4a, the match to 3-hydroxy butanoic acid is fairly good except there is no >C=O band in my spectrum at approximately 1750 cm-1. In fact, this spectrum had the presence of the broad carbon bands near 1340 and 1600 cm-1 that were interfering with my search so I eliminated them manually, and a small feature at ~1750 cm-1 may have been buried in the broad carbon band that I subtracted. Figure 4a is an example that illustrates the hazards of this kind of operation. In addition, my spectrum also shows a broad doublet above 3000 cm-1, which indicates the presence of residual water in the deposit.
Figure 4bi shows the best match to a copolymer of styrene isoprene, which was found in SearchIt but not IDExpert. In IDExpert, the sharp band at approximately 1000 cm-1 was never picked up, probably because it is sharp and at low intensity and would thus have a low contribution to the HQI. In addition, a sharp band near 1660 cm-1 was also missed. However, it is well known that many aromatics have a doublet at 1000–1040 cm-1 and a CH stretch near 3060 cm-1 and 1660 cm-1 often indicates the presence of olefinic >C=C<. Note that SearchIt found the copolymer of styrene isoprene on its first hit. In addition, the relative intensities of these bands at 1000 and 1660 cm-1 varies between my spectrum and that in the database, presumably because of the relative concentrations of the aromatic to olefinic species.
Figure 4bii shows the second spectrum observed in the deposit of this vial and identified it as Sepisol Fast Blue. It turns out that the vials from this vendor were colored, and a comparison of this spectrum in Figure 3bii to that of the colored vial (not shown), the spectrum of the colored vial matched a combination of that of PP and that of the spectrum of Sepisol Fast Blue.
Figure 4c shows a “best” match to the spectrum of the third vendor to a mixture of poly (N-vinyl pyrollidone) and naphthalene 1,3,6 trisulfonic acid trisodium salt. Although the match is not perfect, the prominent bands in the composite spectrum do overlap with the prominent bands in the query spectrum. Are my bands broadened by disorder in the polymer phase and interactions with the smaller molecule? It is hard to say, but it is certainly a possibility. This example is a case where liquid chromatography–mass spec- trometry (LC–MS) or gas chromatography–MS (GC–MS) could be helpful in determining the origin of this contaminant.
Why Are These Molecules Present in the PP vials?
Additives are added to polymers for many reasons, which include antioxidant functionality, gas barrier, physical flexibility, and other reasons. The selection of the additive is dictated by the desired final properties of the material. I am not a polymer engineer so I cannot say what could be the reason for finding the products that we did in these saline solutions. However, in the future, after we have analyzed the deposits from all of the solutions, we may see a recognizable pattern.
An Example Whose Origin is Clear
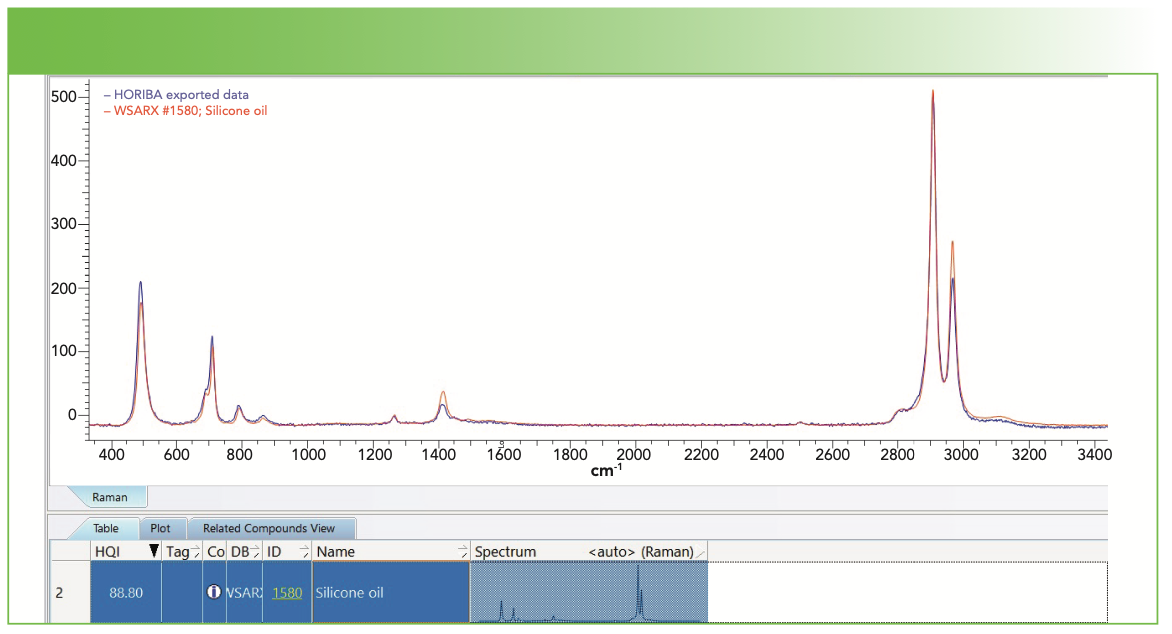
In this article, there is not enough space to review and analyze the total data set. However, I want to show one example that illustrates the identification of a deposit whose presence I do understand. Figure 5 shows the result of a search in the KIA software of a spectrum deposited from a vial of the third vendor that had been exposed to the phosphate buffer at 100 °C. The match is excellent for a silicone oil. In fact, I have a spectrum of a solid silicone (not shown) whose spectral peaks match just as well, but maybe with different relative intensities. I suppose the difference between solid silicone and silicone oil could be simply the molecular weight, and we know that the Raman spectrum is not very sensitive to molecular weight. We also know that in a solid pellet that has presumably been extruded from the melt, there can be molecular orientation that can affect the relative intensities. Can we make sense of the presence of silicone oil in the deposit? Of course! It is often added as a release agent during a molding or extruding operation.
FIGURE 5: Spectrum of a deposit from a vial of the third vendor exposed to phosphate buffer at 100 0C (blue spectrum) overlaid with a match to silicone oil by KIA (red spectrum).
Summary
In an effort to evaluate the potential of Raman microscopy to identify extractables and leachables from polymers, I collected spectra from three sets of polypropylene centrifuge vials from three vendors exposed to 10 different solutions designed to tease out material hiding in the polymer matrix. I examined deposits, for the most part, only from saline solutions. I have described how I collected the spectra, and then how I prepared them for analysis in the KIA software. It is not always clear why particular molecules are being detected, but I picked one example where its presence as a release agent was clear.
The real question is, why bother with all of this? The standard analytical method for identifying extractables and leachables is liquid or gas chromatography combined with mass spectroscopy (LC–MS or GC–MS). What Raman can do that those standard techniques cannot is identify minute amounts of material—typically 1–2 μm2 × 1–5 μm deep. And there are no waste products from the analysis that need to be disposed of. Is the information the same? Maybe yes and maybe no. The measurement is done on intact material whereas MS tears the molecule apart. There may be cases where information is lost in tearing the molecule apart.
From my discussions with Mark Witkowski, I understand that the standard LC–MS and GC–MS techniques have difficulty identifying inorganic materials. In contrast, Raman spectroscopy is equally active for inorganic and organic compounds. In my column last November, where I discussed spectral identification, I showed the excellent identification of a particle of WO3 (4).
For the polymer engineer who is selecting which additives to put in her product, a Raman microscope measurement could be an easy way to determine the safety of the selected additive.
References
(1) General Chapter <1663> “Assessment of Extractables Associated with Pharmaceutical Packaging /Delivery Systems,” in United States Pharmacopeia 43–National Formulary 38 (United States Pharmacopeial Convention, Rockville, MD, 2020).
(2) General Chapter <1664> “Assessment of Leachables Associated with Pharmaceutical Packaging /Delivery Systems,” in USP 43–NF38 (United States Pharmacopeial Convention, Rockville, MD, 2020).
(3) KnowItAll, Wiley.
(4) F. Adar, Spectroscopy 36(11), 8–13 (2021).
Fran Adar is the Principal Raman Applications Scientist for Horiba Scientific in Edison, New Jersey. Direct correspondence to: SpectroscopyEdit@mmhgroup.com. ●


Raman Reveals Rare Ribbeck Meteorite Clues About Ancient Solar System
May 28th 2025A team of scientists in Poland has unveiled the first detailed structural and magnetic analysis of the Ribbeck meteorite, a recently recovered space rock classified as an aubrite. Using Raman spectroscopy, X-ray diffraction, and advanced magnetic testing, researchers revealed the meteorite's unique mineralogy and its connection to deep space conditions.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.
Multi-Analytical Study Reveals Complex History Behind Ancient Snake Motif in Argentine Rock Art
May 22nd 2025A recent study published in the Journal of Archaeological Science: Reports reveals that a multi-headed snake motif at Argentina's La Candelaria rock shelter was created through multiple painting events over time.
New Deep Learning AI Tool Decodes Minerals with Raman Spectroscopy
May 21st 2025Researchers have developed a powerful deep learning model that automates the identification of minerals using Raman spectroscopy, offering faster, more accurate results even in complex geological samples. By integrating attention mechanisms and explainable AI tools, the system boosts trust and performance in field-based mineral analysis.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)