Factors Determining Sensitivity in ICP-MS
Special Issues
Overview of ICP and ICP-MS
Inductively coupled plasma–mass spectrometry (ICP-MS) is renowned for its phenomenal sensitivity as an elemental analyzer. It is the most sensitive elemental spectrometric technique, with detection limits in the low parts-per-trillion or even parts-per-quadrillionrange for many elements. This article examines the reasons for this sensitivity and considers how it might eventually be further improved.
There are basically three types of commercially available inductively coupled plasma–mass spectrometry (ICP-MS) instruments that differ mainly in the type of mass analyzer they use. They are quadrupole, magnetic sector field (also called high resolution ICP-MS), and time of flight. Of these, quadrupole ICP-MS is by far the most commonly used, and this article will be limited to this type of instrument. Most of the relevant principles, however, generally apply to other mass analyzer types as well.
The ICP-MS instrument consists of four main components. The inductively argon coupled plasma ICP serves as the high temperature ion source. The ICP essentially is the same as that used in ICP optical emission instruments with one important difference — the plasma in optical devices is primarily an atom source whereas in a mass spectrometer it produces ions. The interface and ion optic components extract the ions from the plasma at atmospheric pressure and focus them into the mass analyzer, which is at high vacuum. The mass analyzer (quadrupole in this discussion) separates the ions based on their mass-to-charge ratio (m/z) and delivers them sequentially to the detector. The detector is an electron multiplier that generates an electrical pulse to the counting circuitry for each ion that strikes it. Most newer quadrupole ICP-MS instruments also include a collision–reaction cell between the interface and analytical quadrupole. The function of this cell is to remove polyatomic (molecular) interferences before mass analysis. Some loss of signal can occur in this cell due to collisions between analyte ions and gas in the cell.
The argon ICP is a highly efficient ion source. Most metals are ionized at 80 to >95% efficiency, while a few high ionization potential metals such as mercury have ionization efficiencies as low as 40%. Even nonmetals such as sulfur, phosphorus, silicon, and all of the halogens except fluorine are sufficiently ionized to allow for highly sensitive measurement. Only five elements cannot be directly measured by ICP-MS: hydrogen, helium, fluorine, neon, and argon. The first four are not ionized because their first ionization potentials are higher than that of argon, and the last argon is not measurable in an argon plasma.
Let's examine the actual efficiency of a typical quadrupole ICP-MS system. We'll use 1 part per trillion (ppt) as an example detection limit, though some elements will be lower, some higher. A 1-ppt (w/v) concentration is equivalent to 1 ng/L or 10-9 g/L. The equivalent number of atoms (ions) will depend on the atomic weight. Applying the Avogadro constant (6.02 X 1023 atoms/mole), 1 ppt is ~6.68 X 1013 atoms of Be (atomic mass 9) or ~2.53 X 1012 atoms of U (atomic mass 238) per liter. Using a convenient nebulizer flow rate of 1 mL/min would result in between 1.1 X 109 and 4.2 X 107 atoms per second per ppt being introduced to the instrument at the nebulizer. ICP-MS sensitivity is commonly measured in terms of millions of counts per second at the detector per ppm introduced to the nebulizer (Mcps/ppm, also called MHz/ppm). This value varies depending on the element as well as instrument design and operating conditions but is typically ~100–500. So if we work back to our 1-ppt example, this translates to 100–500 cps/ppt. Using the calculation above for the number of atoms introduced to the nebulizer (we'll use an average value of 108 ) for a 1-ppt solution, we can calculate the efficiency of generating a pulse at the detector from a given atom introduced to the nebulizer as 100/108 or 10-6 . About one in a million atoms introduced to the nebulizer actually reach the detector. Where do the rest go? If we examine all of the components of a typical ICP-MS system (Figure 1), we will see that there are inefficiencies (some intentional, some unavoidable) at each stage.
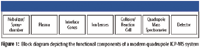
Figure 1: Block diagram depicting the functional components of a modern quadrupole ICP-MS system in order from left to right.
Table I shows the efficiencies that can be assigned to the various stages of ICP-MS analysis in very general terms.
Multiplying the efficiencies in Table I results in an overall estimated efficiency of about 0.00002% (1 in half a million), which is in general agreement with the estimate based on measured response. So, the most sensitive elemental analyzer we have is only about 0.00002% efficient. What if anything can we do about that? And, do we really need to do anything? There are two schools of thought on whether we need to further improve ICP-MS sensitivity. The first school, typified by the commercial service laboratories, would generally say that 1-ppt detection limits are good enough for most applications, and that in fact it is very difficult to routinely produce blanks at that level for many elements. Therefore the actual method detection limits are not instrument limited but are limited by the cleanliness of the sample preparation and laboratory environment.
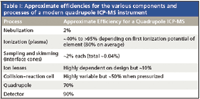
Table I: Approximate efficiencies for the various components and processes of a modern quadrupole ICP-MS instrument
The other school may be looking for sample detection limits below 1 ppt but more commonly is trying to compensate for very small samples or analyte loss during sample preparation or dilution. These are typically research laboratories that can better afford to spend the time necessary to produce sufficiently clean blanks. In any event, the quest for more sensitivity is a real one and has resulted in significant improvements over the years.
Early instruments typically were capable of <1 MHz/ppm sensitivity compared with 100–500 MHz/ppm or higher today. A close look at Table I shows a number of areas where there is significant room for improvement as well those where there is not much to be gained. Most of the opportunities lie in the processes of transporting the sample into the mass spectrometer.
The development of high efficiency or direct injection or total consumption nebulizers in which there is no spray chamber or spray chamber drain permits virtually 100% of the nebulized sample to be introduced to the ICP torch. However limitations in the ability of current ICP plasmas to tolerate increased sample load limit these applications to very low flow, and therefore the total amount of sample introduced to the plasma may not be significantly higher. As a result, total consumption nebulizers have more utility in applications where the sample flow rate or volume is very small rather than in improving instrument sensitivity.
Sampling and skimming efficiency are also quite low (~2% each), again for practical reasons. The sampler and skimmer cones make up the interface between the hot plasma operating within atmospheric pressure and the cool, high-vacuum regions of the mass spectrometer. Through two stages of pressure reduction, the pressure is reduced from ~1 bar outside the sampler cone to ~10-7 bar immediately behind the skimmer. This reduction is achieved through the removal of primarily argon from the plasma and to a much lesser degree sample matrix components, mainly from water. A further 10-fold reduction in pressure is achieved via an entrance aperture into the mass analyzer section that is pumped by high-vacuum turbomolecular pumps. Considering the 107 -fold reduction in pressure across the interface, the 4 X 104 loss of sample isn't too bad. Increasing the efficiency of the interface region (sampler and skimmer) requires larger cone orifices, which in turn require significantly larger vacuum pumps to maintain the necessary vacuum. This can be (and is) done, but the cost of the larger pumps is high. Behind the skimmer, most of the plasma gas has been removed, and the neutral plasma has been largely converted to a positively charged ion beam through removal of electrons at the skimmer or first lens. It is at this point, the ion lens region, that the best opportunity for increasing the efficiency exists. The function of the ion lenses is to focus the ion beam into the mass spectrometer with minimal loss while preventing the transmission of photons and neutral particles that can cause elevated background (noise) if they reach the detector. The ion lenses take advantage of the fact that positively charged ions can be focused and steered by electrostatic fields that have no effect on photons and neutrals. Because of this, the detector can be positioned so that it is not in line of sight of the plasma and the ions steered to the detector.
Two common approaches are taken. The first is to insert a disk in the direct ion path between the interface and quadrupole. This "photon stop" or "shadow stop" serves to physically block light and neutral particles from reaching the detector. This approach was used on early R&D and first-generation instruments in the 1980s. The analyte ions must be defocused around the stop and then refocused behind it. This is a simple but inefficient process that causes significant discrimination against low-mass ions and results in loss of sensitivity in general.
Alternatively, more-modern instruments that locate the quadrupole and detector off axis from the initial ion optics can achieve the same reduction in photons and neutrals while maintaining higher ion transmission across the mass range. This is achieved by using special ion optical components, often called chicane or Omega (Agilent Technologies) lenses, which serve to deflect the ion beam while maintaining ion focus as much as possible. Improvements in ion optics have been responsible for much of the increase in ICP-MS sensitivity over the years, and there is still room for more improvement. Beyond the ion lenses lies the collision cell in instruments so equipped.
The collision cell is a small pressurized cell containing a multipole ion guide to guide the ions through the cell as efficiently as possible. A number of forces within the cell are acting to disrupt the orderly flow of ions from the cell entrance to the cell exit. These are mainly space charge; that is, the mutual repulsion of positively charged ions confined to a small space and collisional scattering due to collisions with the gas in the cell. The multipole's function is to keep the ions focused in the center of the cell and transmit them to the cell exit, while permitting efficient collisions with the cell gas.
Three common multipole configurations are used. They are quadrupole (four rods), hexapole (six rods) and octopole (eight rods). Each type of multipole has its strengths and weaknesses. However as a collision cell ion guide, the octopole has the benefits of highest simultaneous transmission efficiency across the mass range and a wider potential well, which permits more of the space between the rods to be used for collisional purposes. As a result, a collision–reaction cell based on an octopole ion guide can be smaller in volume, reducing gas flow requirements and speeding up cell evacuation. After the ions reach the quadrupole, there is still some opportunity for improvement in sensitivity.
The quadrupole mass filter operates by creating a hyperbolic RF field in which stable ion trajectories are dependent on the mass-to-charge ratio of the ion and the amplitude of an applied RF and DC voltages. The transmission efficiency of the quadrupole is similar to other mass spectrometers in that mass resolution is gained at the expense of sensitivity. Therefore, sensitivity can be improved somewhat if the mass resolution is decreased —that is, the mass peaks are widened. The danger in doing this is related to a characteristic called abundance sensitivity. Abundance sensitivity is the net signal from an isotope present at mass M divided by the signal at mass M+1 or M–1 when there is no isotope present at either M+1 or M–1. This value typically is ~107 and is somewhat higher on the low mass (M–1) side. The net effect is that a weak peak adjacent to a strong peak will be affected by the tailing from the larger peak. Better (higher) abundance sensitivity values can be achieved through the use of quadrupole rods adhering to the ideal hyperbolic cross-sectional shape rather than the less expensive and more common cylindrical shape.
Continual research related to optimizing these components of modern ICP-MS instruments has resulted in significant improvements in both high sensitivity and reduced background when compared with early instruments. This trend obviously will continue, though to what end and through what means is difficult to say. After all, there are still a lot of atoms unaccounted for in that 1-ppt solution.
Steven M. Wilbur is with Agilent Technologies, Bellevue, Washington.

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.