The Hyphenation of HILIC, Reversed-Phase HPLC, and Atmospheric-Pressure-Ionization MS
The coupling of HILIC with reversed-phase HPLC and API-MS will become routine in the future, particularly in fields like metabolomics and environmental analysis that regularly involve nontargeted screening of large numbers of compounds in complex mixtures.
Hydrophilic interaction liquid chromatography (HILIC) and reversed-phase high performance liquid chromatography (HPLC) are complementary techniques in the separation of organic molecules with a broad band of polarity. Both separation variants can be operated easily with volatile and water-miscible solvents (for example, ammonium acetate aqueous solution and acetonitrile). Thus, a hyphenation of both liquid chromatographic techniques with atmospheric pressure ionization (API) and mass spectrometry (MS) seems to be a consequent step.
In recent years, there has been an increased necessity to collect information in a single analysis about a huge number of compounds present in complex mixtures. The so-called "entire component" analysis is particularly required in growing research fields such as metabolomic or environmental analysis that follow a nontargeted approach, in which no hypothesis is formulated about the compound to investigate.
These studies have prompted, and are still prompting, the development of new analytical methods and settings to investigate compounds in a wide range of polarity. In this context, high performance liquid chromatography (HPLC), one of the most powerful separation techniques based on the different physicochemical properties of the compounds, is being widely used. In general, one-dimensional chromatography, in which the separation is achieved with a single column, is not enough to resolve complex mixtures. The hyphenation of two or more columns with different retention mechanisms in multidimensional chromatography is an efficient approach, and was initially developed for proteome analysis (1). In this regard, the orthogonal selectivity of the stationary phases is one of the most important parameters for an efficacious hyphenation. Hydrophilic interaction liquid chromatography (HILIC) is a fairly new technique for the analysis of polar compounds. Its increasing popularity is mainly because of the complementary selectively to the well-known reversed-phase HPLC, which is extensively used for the analysis of hydrophobic analytes. Recent studies have presented interesting on-line coupling of reversed-phase HPLC with HILIC for the analysis of both apolar and polar compounds, such as proteins, drugs, and metabolites in natural samples. Because of the complexity of the mixtures, integration of HPLC systems with atmospheric-pressure-ionization (API) mass spectrometry (MS), used as an identification method based on the molecular weight of the analytes, seems to be preferred. Highly accurate mass spectrometers can be used to obtain empirical formulas for molecules up to 1000 Da and using tandem MS further leads to structural information by molecular fragmentation.
Here, we discuss the principles of HILIC and reversed-phase HPLC, the recent advancements of HILIC×reversed-phase HPLC–API-MS (orthogonal) and HILIC–reversed-phase HPLC–API-MS (linear) hyphenation for the analysis of complex mixtures and its future perspectives.
HILIC and Reversed-Phase HPLC
Reversed-phase HPLC is among the most popular liquid chromatographic techniques, in which the separation is mainly based on hydrophobic interactions between the solutes and the hydrophobic stationary phase; thus, apolar or weakly polar compounds are preferentially retained. Polar and hydrophilic compounds are hard to analyze by classical reversed-phase stationary phases and they are usually eluted near the hold up volume of the column. Appropriate elution conditions with high water content or ion-pair reagents have been developed for the retention of polar analytes. Despite this, such investigations with low-organic-content eluent may cause several problems because of the risk of folding for the hydrophobic alkyl chains and the expulsion of eluent from the pore spaces. Alternatively, polar endcapped reversed-phase stationary phases are commonly used; stationary phases with embedded polar groups (the so-called "aqueous" phases), which are stable in high water content, are also used. In several cases, the high water content in the mobile phase, required for the retention of polar analytes, decreases the sensitivity in API-MS because of the inappropriate ionization conditions (2).
In recent years, HILIC has become the technique of choice for the analysis of hydrophilic and ionic solutes. Although the separation principle has been known since the 1970s, the number of applications exponentially increased after Alpert's seminal paper in 1990 (3). HILIC is considered a variation of normal-phase liquid chromatography because both techniques have polar stationary phases in common. The mobile phase differs because it is a water–water-miscible organic solvent as in reversed-phase HPLC. In general, a water–acetonitrile mixture with the addition of buffer salts is used, and elution is promoted by increasing the water content in the mobile phase. Therefore, the starting setting in HILIC requires a high percentage of organic solvent. In these conditions, a creation of a water-enriched layer partially immobilized on the surface of the stationary phase has been demonstrated. The retention in HILIC is discussed as a mixed-mode mechanism, in which partitioning of the analyte between the organic-rich mobile phase and the water-enriched layer is regarded as the main retention mechanism. However, other important interactions such as hydrogen bonds, electrostatic interactions, and dipole–dipole interactions contribute to the HILIC separation (4).
As an alternative to HILIC, porous graphitic carbon (PGC) chromatography also has shown good capacity and selectivity for the analysis of polar compounds, particularly for isomer separation (5). PGC has a flat, highly crystalline surface, which leads to retention mechanisms that are different from those observed on silica-based bonded phases. The retention mechanism is regarded as a combination of dispersive hydrophobic interactions between the analyte–mobile phase and analyte–graphitic surface and charge-induced interactions of polar analytes with the polarizable surface of the graphite (6). These two types of interaction (dispersive and polar retention) explain the capacity of PGC to also retain hydrophobic compounds. The differences in behavior between PGC and other stationary phases can be effectively exploited in two-dimensional liquid chromatography (2D-LC) systems.
As a general rule, there is no universal choice between reversed-phase HPLC and PGC; in some publications, the first method achieves better separations than the latter, in other cases, the latter is preferred. On the other hand, only a few comparative investigations between HILIC and PCG have been reported thus far (7). However, the higher water content in the mobile phase in PGC chromatography leads to a dramatically lower sensitivity in API-MS detection with respect to HILIC (6).
HPLC Orthogonality
To increase the resolution and peak capacity for "entire-component" analysis of complex samples, the hyphenation of different chromatographic separation modes has proven to be a successful strategy. When doing so, the selection of orthogonal separation conditions is of primary importance, to achieve a maximum difference in selectivity between two separations. Numerous papers report excellent orthogonality between HILIC and reversed-phase HPLC. Ideally, compounds that are strongly retained in reversed-phase HPLC are almost not retained at all in HILIC (8,9). A recent study comparing reversed-phase HPLC, HILIC, and PGC phases for glycan analysis has shown that PGC has intermediate features between HILIC and reversed-phase HPLC (5). The same study concluded that HILIC is the chromatographic method of choice for the analysis of glycans. In the case of complex glycan samples, for which a combination of chromatographic methods may be necessary, the coupling of HILIC to reversed-phase HPLC would lead to the highest degree of orthogonality (5).
How to Hyphenate HILIC with Reversed-Phase HPLC On-Line
HILIC–reversed-phase HPLC is attractive as a hyphenated system for "entire-component" analysis because, in addition to the orthogonality of the two methods, common solvents are used: Both reversed-phase HPLC and HILIC employ water and acetonitrile, which are readily compatible with API sources for MS detection.
In theory, off-line coupling of reversed-phase HPLC and HILIC can be performed simply through collection of the flow-through from the first separation and then reinjection into the second system. Off-line coupling requires simple equipment and allows for the use of columns that are not compatible in terms of solvents. In routine laboratory analysis, however, off-line collection is avoided because it is time-consuming and often results in significant sample loss. Generally, on-line coupling is more desirable for 2D-HPLC. In this case, a column-switching technique is extensively applied, in which a short column is utilized on-line for trapping and transferring selected fractions or all fractions from the first-dimensional to the second-dimensional analytical column. The former method is called "heart-cutting," and the latter is called "comprehensive LC×C" mode (2).
In HILIC–reversed-phase HPLC, the heart-cutting technique is widely employed. All the compounds eluted near the hold-up volume of the first column are transferred to the second column. Despite the use of the same solvents in both modes, the starting condition in HILIC requires high organic content in the mobile phase, but in reversed-phase HPLC high water content is required. This solvent strength incompatibility must be considered in the development of a new chromatographic setting. In the following sections, we describe three general and modern strategies for on-line HILIC–reversed-phase HPLC separation. Most of the other settings reported in literature can be considered a modification of these.
On-Line Orthogonal HILIC–Reversed-Phase HPLC System with Two Detectors
In a recent study, the simultaneous separation of hydrophilic and hydrophobic compounds was reported for "entire component" analysis using an on-line HILIC–reversed-phase HPLC system with two detectors (10). The developed method was applied for the analysis of a traditional Chinese medicine extract, allowing the separation of approximately 200 species.
This system was based on two HPLC modules with binary pumps, coupled with two UV detectors and equipped with a HILIC column and a C18 column. The two columns were hyphenated through an interface with two valves, an isocratic pump and a C18 solute transfer column (column T). The analytical process included three steps, which are represented in Figure 1.
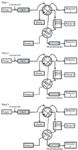
Figure 1
Step 1: Sample loading. The sample was injected onto the HILIC column. The nonretained hydrophobic solutes were eluted at the hold-up time and then were flowed into a mixer in which the eluent was "weakened" with the high-aqueous transfer solvent delivered by the isocratic pump.
Step 2: Compound trapping. The 10-port valve was switched so that the hydrophilic solutes were separated on HILIC column and detected by detector 1. At the same time, the solutes in the mixer were continually eluted by the transfer solvent and trapped into column T.
Step 3: HILIC and reversed-phase HPLC analysis. This step began with the six-port valve switch. The hydrophobic solutes trapped on column T were back-flushed into the C18 column to start the reversed-phase separation and recorded with detector 2. Meanwhile, the hydrophilic solutes were continuously separated on the HILIC column.
Hence, the solutes were divided on-line into two parts based on their hydrophobicity and separated simultaneously on the relevant columns. However, despite the high degree of selectivity that was achieved, this setting presents the serious drawback of requiring two detectors. Because it would be too expensive for most laboratories, this system is not suitable for hyphenation with API-MS for routine analyses.
On-Line Orthogonal HILIC–Reversed-Phase HPLC System with API-MS Detector
In metabolomic and proteomic analysis, LC–API-MS methods are preferred because of the high sensitivity and selectivity of MS detection. In particular, ion-trap (IT) or time-of-flight (TOF) analyzers for MS have shown high sensitivity in scan mode, which is needed for the "entire component" analysis of unknown compounds.
A HILIC–reversed-phase HPLC–API-MS system for the analysis of drugs and their metabolites in blood samples was reported recently (11). The system consisted of two HPLC modules with binary pumps, equipped with a zwitterionic HILIC column and a C18 column, hyphenated through an interface with two valves. The analytical sequence included three steps, which are represented in Figure 2.
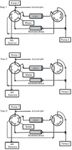
Figure 2
Step 1: Compound trapping. The sample was injected onto the HILIC column. The polar compounds were then retained at the head of the HILIC column and the nonpolar fraction moved toward the reversed-phase column. After the hydrophobic compounds arrived at the reversed-phase column, the second binary pump was activated to decrease the acetonitrile percentage of the mobile phase to 10% and trap the analytes.
Step 2: HILIC analysis. Through the switching of the valves, the autosampler and the reversed-phase column were disconnected from the LC system. The flow rate of the reversed-phase mobile phase was decreased and directed to waste, and the desorption of the polar fraction on the HILIC column and its analysis were carried out in gradient mode.
Step 3: Reversed-phase HPLC analysis. Next, valve 1 was switched to connect the reversed-phase column to the MS detector and the HILIC column was cleaned up in backflushing mode. The reversed-phase fraction was analyzed by a conventional gradient elution increasing the acetonitrile content in the mobile phase.
Linear On-Line HILIC–Reversed-Phase HPLC System with API-MS Detector
On-line HILIC–reversed-phase HPLC systems are based on valve switching. These techniques are quite sophisticated for routine analysis in manufacturing laboratories. Indeed, in the settings discussed above, the two chromatographic systems were hyphenated with special interfaces to overcome the solvent strength incompatibility between HILIC and reversed-phase HPLC.
A simple and effective approach for a linear on-line coupling of HILIC to reversed-phase HPLC without the use of valves was recently described (12). In this case, the first dimension is reversed-phase HPLC and the second is HILIC. A key aspect of this approach was the use of a 2-mm i.d. reversed-phase column and a 4.6-mm i.d. HILIC column. The analytical sequence included three steps, which are represented in Figure 3.
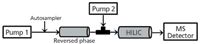
Figure 3: Schematic representation of a linear on-line HILICâreversed-phase HPLC system with one API-MS detector.
Step 1: Compound trapping. The sample was injected through onto the reversed-phase column. The hydrophobic compounds were then retained and the polar compounds were directed toward the HILIC column. The mobile phase from the reversed-phase column was connected via a tee-piece to the second pump and the organic content in the mobile phase was increased (for example, ≥80%) to trap the polar fraction onto the HILIC column.
Step 2: HILIC analysis. The polar analytes were eluted from the HILIC column by increasing the water content in the mobile phase of the second pump. At the same time, the high water content in the first dimension ensured the retention of the hydrophobic analytes.
Step 3: Reversed-Phase HPLC analysis. Subsequent to the HILIC analysis, the water content in the HILIC eluent was maintained around 50%. The reversed-phase separation started in gradient mode. All of the hydrophobic compounds passed through the HILIC column without retention.
Besides the absence of valves, this system also presents the advantage of using a single detector module. This linear on-line HILIC–reversed-phase HPLC system was reported coupled with an API-TOF-MS detector. The use of a high percentage of organic solvent increases the MS ionization, with ensuring higher sensitivity. However, the high flow rate required for diluting the eluent coming from the reversed-phase column before it enters the HILIC column may represent a serious hindrance for MS hyphenation.
Perspectives
Mass spectrometers have become more robust, specific, and sensitive. MS allows users to obtain empirical formulas (by accurate MS) and structural information (by tandem MS) for single molecules (13). This physicochemical information is essential for the identification and characterization of molecules (along with the possible use of nuclear magnetic resonance spectroscopy or infrared spectroscopy).
However, chromatography extends this information by the parameter hydrophobicity vs. retention time index. The latter can be correlated with the logarithm of partition coefficient (log P) or, in case of ionic analytes, with the distribution coefficient (log D).
In a newly progressed strategy of nontargeted screening a large amount of information is needed for the characterization of unknown species. Application fields include metabolomics (14), authenticity analysis as with food and beverages, or risk potential in wastewater monitoring.
Although the development of software with improved performance for data interpretation, as well as databases, is still necessary, HILIC–reversed-phase HPLC–API-MS coupling will become a routine technique in the future.
Acknowledgment
This work was in part supported by a financial grant (PDOK-75-10) from the Bayerische Forschungsstiftung (BfS) that covered Dr. Greco's fellowship.
Giorgia Greco is with the Analytical Research Group, Wissenschaftszentrum Weihenstephan, Technische Universität München, in Freising–Weihenstephan, Germany.
Thomas Letzel is with the Competence Pool Weihenstephan, associated with Technische Universität München, in Weihenstephan, Germany. Please direct correspondence to: t.letzel@wzw.tum.de.
References
(1) C.R. Evans and J.W. Jorgenson, A nal. Bioanal. Chem. 378, 1952–1961 (2004).
(2) Y. Wang, R. Lehmann, X. Lu, X. Zhao, and G. Xu, J. Chromatogr. A 1204, 28–31 (2008).
(3) A.J. Alpert, J. Chromatogr. 499, 177–196 (1990).
(4) G. Greco, S. Grosse, and T. Letzel, J. Chromatogr. A. 1235, 60–67 (2012).
(5) M. Melmer, T. Stangler, A. Premstaller, and W. Lindner, J. Chromatogr. A 1218, 118–123 (2011).
(6) L. Pereira, LCGC N. America 29(3), 262–269 (2011).
(7) C. West, C. Elfakir, and M. Lafosse, J. Chromatogr. A 1217, 3201–3216 (2010).
(8) L. Hu, M. Ye, X. Jiang, S. Feng, and H. Zou, Anal. Chim. Acta 598, 193–204 (2007).
(9) M. Gilar, P. Olivova, A.E. Daly, and J.C. Gebler, Anal. Chem. 77, 6426–6434 (2005).
(10) Y. Wang, X. Lu, and G. Xu, J. Sep. Sci. 31, 1564–1572 (2008).
(11) A. Thomas, J. Déglon, T. Steimer, P. Mangin, Y. Daali, and C. Staub, J. Sep. Sci. 33, 873–879 (2010).
(12) S. Louw, A.S. Pereira, F. Lynen, M. Hanna-Brown, and P. Sandra, J. Chromatogr. A 1208, 90–94 (2008).
(13) J.S. Patrick, K. Siek, J. Binkley, V. Artaev, and M. Mason, LCGC N. America and Spectroscopy supplement: Current Trends in Mass Spectrometry , 18–25 (May 2011).
(14) T. Sana, S. Fischer, and S.E. Tichy, LCGC N. America and Spectroscopy supplement: Current Trends in Mass Spectrometry , March 2011, 12–17 (2011).

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.