Identification of Organic Additives in Nitrile Rubber Materials by Pyrolysis-GC–MS
Special Issues
Nitrile rubber materials were studied using flash analytical pyrolysis-GC–MS to demonstrate that this technique is a good tool to identify the additives in nitrile rubber.
The criteria for assessing the quality of rubber materials are the polymer or copolymer composition and the additives. These additives include plasticizers, extender oils, carbon black, inorganic fillers, antioxidants, heat and light stabilizers, processing aids, cross-linking agents, accelerators, retarders, adhesives, pigments, smoke and flame retardants, and others. Determination of additives in polymers or copolymers generally requires the extraction of these substances from the matrix as a first step, which can be challenging, and the subsequent analysis of the extracted additives by gas chromatography (GC), GC–mass spectrometry (MS), high performance liquid chromatography (HPLC), HPLC–MS, capillary electrophoresis, thin-layer chromatography, and other analytical techniques. In the present work, nitrile rubber materials were studied using direct analytical flash pyrolysis hyphenated to GC and electrospray ionization MS in both scan and selected ion monitoring modes to demonstrate that this technique is a good tool to identify the organic additives in nitrile rubber.
Commercial plastics and rubbers always contain low-molecular-weight additives. These compounds are essential in polymer or copolymer processing and in ensuring the end-use properties of a polymer or copolymer. Additives can improve or modify the mechanical properties (fillers and reinforcements), modify the color and appearance (pigments and dyestuffs), give resistance to heat degradation (antioxidants and stabilizers), provide resistance to light degradation (UV stabilizers), improve the flame resistance (flame retardants), improve the performance (antistatic or conductive additives, plasticizers, blowing agents, lubricants, mold release agents, surfactants, and preservatives), and improve the processing characteristics (recycling additives) of polymers or copolymers (1,2). Some of these additives accumulate in the environment and affect our health and the environment (3). Knowledge of additives is important for evaluating the environmental impact and interaction of polymeric materials, investigating long-term properties and degradation mechanisms, verifying ingredients, investigating manufacturing problems, qualifying control polymeric materials, identifying odorants, avoiding workplace exposure, and insuring safety of food packaging and medical products (3). Identification of polymer or copolymer additives is also desired if competitor products are investigated. The quantification of additives is important for quality control and troubleshooting of the manufacturing processes. Both identification and quantification are difficult tasks because there are a wide variety of different additives, usually mixtures of additives are used, and the added amount is often low and can be further decreased because of degradation (4,5).
Most analytical methods reported for the determination of polymer or copolymer additives require previous extraction of the additives from the polymeric material. For this purpose, widely used sample preparation techniques such as liquid extraction with various solvents, Soxhlet extraction, ultrasonic-assisted extraction, and pressurized liquid extraction like microwave assisted extraction, accelerated solvent extraction, supercritical fluid extraction, and extraction processes using autoclaves are used (3–6,9). All of these methods are very laborious and time-consuming. Subsequent analysis of the extracted additives has been performed using thin-layer chromatography, supercritical fluid chromatography, gas chromatography (GC), high performance liquid chromatography (HPLC), and capillary electrophoresis (CE), or their conjunction with mass spectrometry (GC–MS, HPLC–MS, and CE–MS) (5–9). Infrared or UV spectroscopic investigations of polymers or copolymers, particularly in the form of a thin film are only applicable for a sample containing just a single additive or if determination of a sum-parameter is sufficient (6).
Several reports on the use of matrix-assisted laser desorption–ionization mass spectrometry (MALDI-MS) for the detection of additives in plastic samples exist (5). Nevertheless, these methods require fine grinding of the polymer or extraction and dissolution of the sample and subsequent analysis of the extract. Besides investigations on thin polymer films using time-of-flight secondary ion mass spectrometry (TOF-SIMS), polymer additives have been detected directly in polymer samples by laser-MS (5). The direct MS methods are the focus of current research for identification of solid polymeric materials (5).
The thermo-analytical techniques, such as thermogravimetric analysis or temperature-programmed analytical pyrolysis, specifically take advantage of relatively slow heating, particularly in combination with appropriate detection modes like thermogravimetric-MS, thermogravimetric-Fourier transform infrared spectroscopy, and temperature-programmed pyrolysis-GC–MS (8,9). Also headspace solid-phase microextraction (3,16) and thermal extraction (2) in conjunction with GC–MS have been applied for the extraction and identification of several common polymer or copolymer additives. In such volatile removal techniques, the additives are usually detected at temperatures below the decomposition temperature of the polymer or copolymer. It is also possible to gain information about additives from flash analytical pyrolysis (8–15). The flash analytical pyrolysis technique hyphenated to GC–MS has extended the range of possible tools for characterization of synthetic polymers or copolymers. Reproducible decomposition products characteristic for the original polymer or copolymer sample are formed under controlled conditions at elevated temperatures (500–1400 °C) in the presence of an inert gas. The pyrolysis products are separated chromatographically using a fused-silica capillary column and are subsequently identified by interpretation of the obtained mass spectra or by using mass spectral libraries (for example, NIST or Wiley). Pyrolysis methods eliminate the need for pretreatment by performing analyses directly on the solid polymer or copolymer sample. The pyrolysis unit is directly connected to the injector port of a gas chromatograph. A flow of an inert carrier gas, such as helium, flushes the pyrolyzates into the fused-silica capillary column. The detection technique of the chromatographically separated compounds is typically MS.
The study of rubbers is the oldest application of the analytical flash pyrolysis-GC–MS technique. In the literature there is a lack of methods for identification of additives in nitrile rubber by this analytical technique. In the present work, nitrile rubber materials have been studied using flash analytical pyrolysis-GC–MS to demonstrate that this technique is a good tool to identify the additives in nitrile rubber.
Experimental
Samples
Membrane of a hydraulic cylinder from the automotive industry and an O-ring seal were used in the investigation.
Apparatus and Analytical Conditions
Approximately 100–200 µg of solid rubber sample was cut out with a scalpel and inserted without any further preparation into the bore of the pyrolysis solids injector and then placed with the plunger on the quartz wool of the quartz tube in the furnace pyrolyzer (Pyrojector II, SGE). The pyrolyzer was operated at a constant temperature of 700 °C. The pressure of the helium carrier gas at the inlet to the furnace was 95 kPa. The pyrolyzer was connected to a 7890A gas chromatograph with a series 5975C quadrupole mass spectrometer (Agilent Technologies Inc.) operated in the electron ionization (EI) mode and in the selected ion monitoring (SIM) mode. A 60 m × 0.25 mm, 0.25-µm df DB-5ms fused-silica capillary column was used. Helium (grade 5.0, Westfalen AG) was used as a carrier gas. The GC conditions were as follows: programmed temperature of the capillary column from 75 °C (1 min hold) at 7 °C/min to 280 °C (hold to the end of analysis) and programmed pressure of helium from 122.2 kPa (1 min hold) at 7 kPa/min to 212.9 kPa (hold to the end of analysis). The temperature of the split–splitless injector was 250 °C and the split ratio was 20:1. The transfer line temperature was 280 °C. The EI ion source temperature was kept at 230 °C. The ionization occurred with a kinetic energy of the impacting electrons of 70 eV. The quadrupole temperature was 150 °C. Mass spectra and reconstructed chromatograms (total ion current [TIC]) were obtained by automatic scanning in the mass range m/z 35–750 u. GC–MS data were processed with ChemStation software (Agilent Technologies) and the NIST 05 mass spectral library (Agilent Technologies).
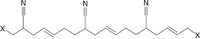
Figure 1: Chemical structure of poly(acrylonitrile-co-butadiene) (nitrile rubber).
Results and Discussion
The commonly used rubbers are natural rubber (polyisoprene), synthetic polyisoprene, polybutadiene, styrene–butadiene copolymers, and nitrile rubber.

Figure 2: Pyrolysis-GCâMS chromatogram (TIC) of the membrane of a hydraulic cylinder from the automotive industry at 700 °C.
Nitrile rubber (poly[acrylonitrile-co-butadiene]) (Figure 1) is a copolymer containing 15–50% acrylonitrile, manufactured by emulsion polymerization of acrylonitrile and 1,3-butadiene. It was invented around the same time as styrene–butadiene copolymers in the German program (at the end of the 1920s) to find substitutes for natural rubber (17). The major applications for this material are in areas requiring oil and solvent resistance. The largest market for nitrile rubber is in the automotive industry because of its solvent and oil resistance. Major end uses are for hoses, fuel lines, O-rings, gaskets, and seals. In blends with poly(vinyl chloride) and poly(acrylonitrile-co-butadiene-co-styrene), nitrile rubber acts as an impact modifier. Some nitrile rubber is sold in latex form for the production of grease-resistant tapes, gasketing material, and abrasive papers. Latex also is used to produce solvent resistant gloves (17).

Figure 3: Pyrolysis-GCâMS chromatogram (TIC) of an O-ring seal at 700 °C.
The analysis of rubber by flash pyrolysis-GC–MS is complex and possible interferences are numerous. Some rubbers contain more than 15 ingredients, some at a low (<1%) level (9). Because of the low concentration of additives and the possible pyrolyzate interferences from the original polymer or copolymer matrix, the evaluation and interpretation of pyrograms requires a lot of time and experience. The peaks obtained for additives are often recognizable as small irregularities within the characteristic pyrogram of the pyrolyzed material. Figures 2 and 3 show the TIC (pyrograms) of both investigated rubber samples obtained after pyrolysis at 700 °C. The degradation products identified by EI mass spectrometry are summarized in Table I. Both investigated samples were identified as nitrile rubber based on the decomposition products like propene, 1,3-butadiene, acrylonitrile, methacrylonitrile, 1,3-cyclopentadiene, 1,4-cyclohexadiene, benzene, toluene, styrene, and benzonitrile. The main feature of the pyrolysis of nitrile rubber is the formation of the monomers 1,3-butadiene (t R = 6.76 min) and acrylonitrile (t R = 6.98 min). The presence of benzonitrile (t R = 11.83 min) in pyrograms is also characteristic for the pyrolysis of nitrile rubber (18). Other substances that appear in pyrograms (Figures 2 and 3) and in Table I, indicated with the letters A to I, have been identified by EI MS as nitrile rubber additives or their thermal degradation products. The mass spectra obtained in full-scan mode and the appropriate chemical structures of the substances are shown in Figure 4. The substance diethylene glycol mono-n-butylether (2-[2-butoxyethoxy]-ethanol, CAS No. 112-34-5) (Figure 2, peak A), identified in the membrane of the hydraulic cylinder from the automotive industry, is a residual solvent from the rubber sample. The substance is known as an excellent coalescing and coupling agent. The identified benzothiazole (Figure 2, peak B) has been formed by the thermal degradation of 2-mercaptobenzothiazole (CAS number 149-30-4). 2-Mercaptobenzothiazole is used as an accelerator for the vulcanization of rubber and as an antioxidant. The identified N-(1-methylethyl)-N´-phenyl-1,4-benzenediamine (N-isopropyl-N´-phenyl-p-phenylendiamine, CAS number 101-72-4) (Figure 2, peak D) is a very effective antioxidant and antiozonant that provides medium to long term protection for all synthetic and natural rubber. Furthermore, N-phenyl-1,4-benzenediamine (Figure 2, peak C) was generated from N-(1-methylethyl)-N´-phenyl-1,4-benzenediamine during the pyrolysis of the nitrile rubber sample.
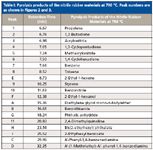
Table I: Pyrolysis products of the nitrile rubber materials at 700 °C. Peak numbers are as shown in Figures 2 and 3.
The examined O-ring was identified as nitrile rubber with high content of the plasticizers bis(2-ethylhexyl) phthalate (CAS number 117-81-7) (Figure 3, peak H, t R = 23.56 min) and 2-ethylhexyl benzoate (CAS number 5444-75-7) (Figure 3, peak I, t R = 25.52 min). The thermal decomposition of the plasticizer bis(2-ethylhexyl) phthalate leads to the formation of 2-ethyl-1-hexene (Figure 3, peak E, t R = 8.73 min), 2-ethyl-1-hexanol (Figure 3, peak F, t R = 12.38 min), and phthalic anhydride (Figure 3, peak G, t R = 18.24 min) during the pyrolysis. The obtained full-scan EI mass spectra of the compounds and the appropriate chemical structures are shown in Figure 4, and the reaction of the thermal degradation of bis(2-ethylhexyl) phthalate is presented in Figure 5. The chemical reaction scheme is consistent with the previously published work (19).

Figure 4: EI full-scan mass spectra at 70 eV and the chemical formulas of additives or substances formed by the thermal decomposition of additives, identified in nitrile rubber materials: (a) diethylene glycol mono-n-butylether, (b) benzothiazole, (c) N-phenyl-1,4-benzenediamine, d) N-(1-methylethyl)-N´-phenyl-1,4-benzenediamine, (e) 2-ethyl-1-hexene, (f) 2-ethyl-1-hexanol, (g) phthalic anhydride, (h) bis(2-ethylhexyl) phthalate, and (i) 2-ethylhexyl benzoate.
SIM mode in MS can be used for detection of substances with known m/z of the molecular or fragment ions and for the quantification. In our investigation, SIM was used to follow the appearance of the ion current curves of the molecular ions m/z 135 of benzothiazole (peak B), m/z 184 of N-phenyl-1,4-benzenediamine (peak C), m/z 226 of N-(1-methylethyl)-N´-phenyl-1,4-benzenediamine (peak D), and of the base fragment ions m/z 70 of 2-ethyl-1-hexene (peak E), m/z 57 of 2-ethyl-1-hexanol (peak F), m/z 104 of phthalic anhydride (peak G), and m/z 105 of 2-ethylhexyl benzoate (peak I). The obtained SIM traces are shown in Figure 6.
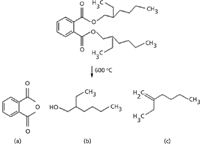
Figure 5: Chemical reaction of the thermal decomposition of bis(2-ethylhexyl) phthalate at 700 °C leading to obtain (a) phthalic anhydride, (b) 2-ethyl-1-hexanol, and (c) 2-ethyl-1-hexene.
Conclusion
The analytical flash pyrolysis hyphenated to gas chromatography and EI mass spectrometry in both scan and SIM modes has proven to be a valuable technique for the identification of organic additives in nitrile rubber materials. This technique allows for the direct analysis of very small solid rubber sample amounts without the need for time-consuming sample preparation. The presented method can be used in R&D, manufacturing processes in the rubber industry, failure analysis, and environmental protection.

Figure 6: SIM traces of additives or their thermal decomposition products, identified in nitrile rubber materials: (a) benzothiazole, (b) N-phenyl-1,4-benzenediamine, (c) N-(1-methylethyl)-N´-phenyl-1,4-benzenediamine, (d) 2-ethyl-1-hexene, (e) 2-ethyl-1-hexanol, (f) phthalic anhydride, and (g) 2-ethylhexyl benzoate.
Acknowledgments
I would like to thank my daughter Maria Kusch, M.A., for her critical reading of the manuscript.
Dr. Peter Kusch is a scientist at the Bonn-Rhine-Sieg University of Applied Sciences in the Department of Applied Natural Sciences in Rheinbach, Germany. Please direct correspondence to: peter.kusch@h-brs.de.
References
(1) R. Gächter and H. Müller in Plastics Additives Handbook, 4th Edition (Hansa Publishers, Munich, Germany, 1993).
(2) M. Herrera, G. Matuschek, and A. Kettrup, J. Anal. Appl. Pyrolysis 70, 35–42 (2003).
(3) M. Hakkarainen in Advances in Polymer Science, Vol. 211, Chromatography for Sustainable Polymeric Materials, A.-C. Albertsson and M. Hakkarainen, Eds. (Springer-Verlag, Berlin, Heidelberg, Germany, 2008), pp. 23–50.
(4) M. Himmelsbach, W. Buchberger, and E. Reingruber, Polym. Degrad. Stab. 94, 1213–1219 (2009).
(5) W. Buchberger and M. Stiftinger in Advances in Polymer Science, Vol. 248, Mass Spectrometry of Polymers – New Techniques, M. Hakkarainen, Ed. (Springer-Verlag, Berlin, Heidelberg, Germany, 2012), pp. 39–68.
(6) S. M. Reiter, W. Buchberger, and C. W. Klampfl, Anal. Bioanal. Chem. 400, 2317–2322 (2011) and references cited therein.
(7) M. Himmelsbach and W. Buchberger, GIT Labor-Fachzeitschrift 52(3), 214–216 (2008).
(8) J.C.J. Bart, J. Anal. Appl. Pyrolysis 58–59, 3–28 (2001) and references cited therein.
(9) J.C.J. Bart in Additive in Polymers, Industrial Analysis and Application (John Wiley & Sons, Chichester, England, 2005), pp. 29–48.
(10) F. Cheng-Yu Wang, J. Chromatogr. A 883, 199–210 (2000).
(11) F. Cheng-Yu Wang, J. Chromatogr. A 886, 225–235 (2000).
(12) F. Cheng-Yu Wang and W.C. Buzanowski, J. Chromatogr. A 891, 313–324 (2000).
(13) F. Cheng-Yu Wang, J. Chromatogr. A 891, 325–336 (2000).
(14) M. Blazsó, Zs. Czégény, and Cs. Csoma, J. Anal. Appl. Pyrolysis 64, 249–261 (2002).
(15) K.D. Jansson, C.P. Zawodny, and T.P. Wampler, J. Anal. Appl. Pyrolysis 79, 353–361 (2007).
(16) R. Rogalewicz, A. Voelkel, and I. Kownacki, J. Environ. Monit. 8(3), 377–383 (2006).
(17) D.F Graves in Rubber in Kent and Riegels Handbook of Industrial Chemistry and Biotechnology, Part 1, J.A. Kent, Ed. (Springer Science + Business Media, New York, New York, 2007), pp. 689–718.
(18) S.-S. Choi and D.-H. Han, J. Anal. Appl. Pyrolysis 80, 53–60 (2007).
(19) J.L. Bove and P. Dalven, The Science of the Total Environment 36(1), 313–318 (1984).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.