IR Spectroscopy Analysis of Disposable Gloves for Residues
Residue can be transferred from gloves to samples at detectable levels, especially in attenuated total reflectance infrared spectra. Some residues can be removed easily by washing and drying the gloves in a manner similar to washing and drying hands.

Gloves are used in the laboratory for two main reasons. The first is to protect the wearer from potentially harmful substances. The second is to protect the sample itself from contamination. If an analyst handles a sample with bare hands, there is a risk of transferring contaminants such as skin cells and oils onto the sample. IR absorption bands from the contaminants can appear in the spectrum and interfere with the analysis of the sample. Sample contamination from residues on gloves might not always be considered, but it can be important.
Gloves can be coated with mold-release agents that provide a powder-free glove that can be stripped easily from the glove formers following fabrication. Release agents can include polypropylene wax, fluorocarbon resin, polydimethylsiloxane, and silicone diol. Slip agents facilitate the stripping and everting of the gloves from the mold, increasing efficiency of manufacture. These can include silicone and oxidized high-density polyethylene (HDPE) (1).
In the analytical laboratory, contamination of a sample directly affects the validity of its analysis. After a sample is contaminated, any data amassed using that sample will be suspect, be it a gravimetric analysis or more sophisticated instrumental techniques such as IR or mass spectrometry (MS). Contamination from handling samples with bare hands can occur, and gloves could be a way to prevent that contamination. However, absorption bands attributed to sample contamination from gloves can be seen in attenuated total reflectance (ATR) IR spectra. Because ATR is a surface analysis technique, sampling approximately a few thousand angstroms into the sample, a small amount of surface contamination can be detected. If the analyst is unaware of the possibility of contamination from gloves, residue from gloves on the sample might appear in the spectra and could be interpreted as a part of the sample.
Research into glove residue contamination has been reported previously. Welker (2) studied glove residue with respect to regulating the amount of particles introduced into clean rooms. He suggested that washing gloves while wearing them could rinse off particles that could otherwise be transferred into the air or onto the sample. Similarly, Sovinski (3) of NASA researched the amount of nonvolatile residue certain brands of gloves leave so as to recommend different types of gloves for different clean room situations, depending upon the stringency of the clean room restrictions and the types of solvents handled while wearing gloves.
Types of Gloves
In a laboratory, there can be several types of gloves available for various purposes. Latex gloves work well for dilute acids and bases, but the rubber is altered easily by organic solvents. In addition, latex allergy can limit their use (4). Nitrile gloves can withstand more organic solvents for longer periods than latex gloves. Nitrile gloves are both more flexible and more resistant to tearing than latex gloves. Vinyl gloves are suitable for the same situations as nitrile gloves; however, they are more prone to ripping. Any of these materials — latex, nitrile, or vinyl — can be used for general laboratory work. Keeping the sample clean is a major concern, for example, when preparing a sample in which trace materials can be detected. Nylon gloves are used more specifically to control the amount of material transferred as well as for heat protection when working with samples to be analyzed in the gas chromatography–mass spectrometry (GC–MS) system. Generally, gloves are chosen based upon the protection they provide.
Experimental
IR spectroscopy was used to analyze several different brands and types of gloves. The spectrometer used was a Thermo Nicolet Magna 550 (Thermo Fisher Scientific, Madison, Wisconsin) with a Golden Gate diamond crystal micro ATR device including a clamping device. Spectra were taken of each glove sample, and also of any residue left behind on the diamond after the glove was removed. In addition, pairs of gloves were worn, washed with standard dish soap or hand soap, removed, and either left in a hood until dry or dried with a paper towel. Spectra of the residues from fingerprints of various laboratory personnel under various conditions were taken by pressing the finger to the crystal and then removing it.
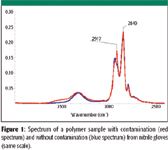
Figure 1
Results and Discussion
Samples can be contaminated by coming into direct contact with gloves. Figures 1 and 2 show an ATR spectrum of a sample with and without contamination from a nitrile glove. The bands at 2917, 2849, 1577, 1539, and 1472 cm–1 are attributed to the contamination. When the gloves were washed, the amount of residue that appeared in the spectra was reduced significantly. In some instances, the gloves left a barely detectable residue, so they could be considered "clean."
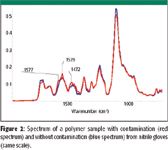
Figure 2
The four types of gloves analyzed by IR for this study and the residues found are listed in Table I.
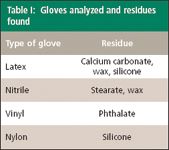
Table I: Gloves analyzed and residues found
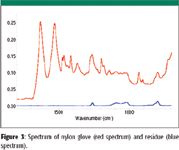
Figure 3
Among other things, calcium stearate is used as a releasing agent for plastic molding powders and as a stabilizer for polyvinyl chloride resins (5). Wax, indicated in IR spectra by a doublet around 710 cm–1, can be a component of the glove itself or a byproduct of the manufacturing process. Phthalates typically are used as plasticizers to soften plastics and make them more flexible (6). Phthalates incorporate themselves into the crystalline structure of most plastics, thereby interrupting the crystal lattice and, as a result, making the plastic more flexible (7). Polymer additives can rise to the surface over time, so older gloves might have more residues than newer gloves. Silicone has many uses, including for lubricants, release agents, "knitting" or finishing oils in woven gloves, and as an ingredient in plastics and rubbers, which might explain its existence as a residue on a glove (8). Figure 3 shows the spectrum of a nylon glove and the silicone residue on the same absorbance scale. The silicone residue appeared to be significant. Figure 4 shows IR spectra of three gloves. The spectra of the two different latex gloves clearly show different levels of calcium carbonate coating. Phthalate can be seen clearly in the spectrum of the vinyl glove.
Figure 5 shows that silicone was the residue left on the ATR crystal from the latex glove with the large amount of carbonate. The carbonate was not detected. However, because the chemical and physical composition of samples could be quite different from the hard, smooth diamond ATR crystal, the contamination that could transfer to a sample might be different from what was left on the crystal.
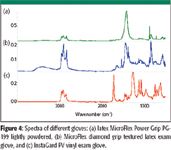
Figure 4
Spectra of residues from fingers also were taken. The expected components would include fatty acids, long-chain fatty acid esters, cholesterol, and squalene (9). Prints from children contain higher levels of relatively volatile free fatty acids, while those from adults have higher levels of less volatile long-chain esters of fatty acids (10). In these experiments, long-chain fatty acids and esters were found from ordinary fingerprints. Calcium stearate and zinc stearate were found in prints from washed and dried hands. After hand lotion was used, silicone residue was found (Figure 6). No detectable cholesterol or squalene was found in any of the residues from fingers.
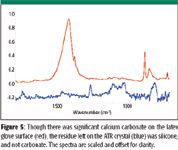
Figure 5
Conclusion
Many gloves used for personal or sample protection can leave an unwanted residue on samples. To prevent this from interfering with the sample analysis, it is important to be aware of what kind of contamination can occur and to take mitigating steps. Washing and drying gloves was found to reduce or eliminate the sample contamination.
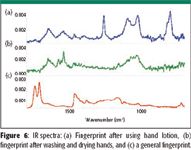
Figure 6
Richard Castino, Laryssa Patti, and K.A. Lee are with Sun Chemical, Carlstadt, New Jersey.
References
(1) G. Wypych, Handbook of Antiblocking, Release, and Slip Additives (ChemTec Publishing, Toronto, Ontario, Canada, 2005).
(2) R.W. Welker, "Using Contamination and ESD Tests to Qualify and Certify Clean Room Gloves." www.vwrsp.com/highlights/pss/articles/qualifyncertify.pdf
(3) M.F. Sovinski, "Contamination of Critical Surfaces from NVR Gloves Residues via Dry Handling and Solvent Cleaning." April 2004. code541.gsfc.nasa.gov/Recent_Publications/04-4_Sovinski.pdf
(4) S. Reddy, "Latex Allergy," American Family Physician, January 1, 1998. www.aafp.org/afp/980101ap/reddy.html, K. Turjanmaa, Contact Dermatitis 17(5), 270–275 (1987).
(5) Merck Index, 1983. p.234. Rahway, New Jersey.
(6) www.chemicalland21.com/industrialchem/plasticizer/DIUNDECYL%20PHTHALATE.htm
(7) "An Overview of The Plasticisation Process," The European Council for Plasticisers and Intermediates, 2007. www.plasticisers.org/index.asp?page=35
(8) Merck Index, (Merck and Co., Inc., Rahway, New Jersey,1983), p. 8341.
(9) K. Asano, C. Bayne, M. Buchanan, and K. Horsman, J. Forensic Sci. 47(4), 18–21 (2002).
(10) M.V. Buchanan, K. Asano, and A. Bohanon, "Chemical Characterization of Fingerprints from Adults and Children," Photonics East`96: International Society for Optical Engineering (SPIE) Conference and Exhibition on Photonic Sensors and Controls for Commercial Applications, Boston, MA, 19–21 Nov. 1996, Office of Scientific and Technical Information (OSTI), US Department of Energy, Report # CONF-961113—14.

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.