Key Errors to Avoid in the Consideration of Fluorescence Quenching Data
Special Issues
Fluorescence quenching is the nonradiative loss of excitation energy from a fluorophore through an interaction with another species, called a quencher. It is a powerful, versatile, and widely used methodology that can, for example, be the basis for chemical and biological assays and sensors, and is useful for probing biomolecular structure. A side effect of this broad scope and utility, combined with the relative technical ease of the experiments, is that the analysis, interpretation, and discussion of fluorescence quenching data is sometimes prone to errors. A correct molecular and spectroscopic interpretation is essential for obtaining useful physicochemical insight from quenching data. This article summarizes several common errors to avoid in the consideration of fluorescence quenching data.
Implying That Dynamic Quenching and Static Quenching Are Specific Mechanisms of Quenching
Dynamic quenching and static quenching are not mechanisms of fluorescence quenching, but rather two classes of mechanisms of quenching. Per the recommendations of the International Union of Pure and Applied Chemistry (1), dynamic quenching is a process that interferes with emission from the excited state of a fluorophore after the excited state has formed. A hallmark of dynamic quenching is thus a change in the observed fluorescence lifetime of the fluorophore. In contrast, static quenching is a process that inhibits the initial formation of the emissive excited state, and there is no apparent change in the fluorescence lifetime. Given that neither terminology implies any other details about how quenching occurs, multiple mechanisms of quenching fit the definitions of both terms.
Equating Collisional Quenching and Dynamic Quenching
Many resources use the quenching of a fluorophore by dissolved oxygen, acrylamide, or halide ions as an illustrative example of dynamic quenching. These instances are also often used as a concurrent example of collisional quenching. The terms dynamic quenching and collisional quenching have thus, unfortunately, become synonymous for some scientists. There are two points that readily debunk this misconception.
First, there is dynamic quenching that is not collisional quenching. Common examples are Förster resonance energy transfer (FRET) systems, where the fluorophore and quencher are attached to the same molecule (or molecular complex), and thus diffuse together. The energy transfer occurs through space, over distances up to approximately 10 nm, and therefore does not require collisions or any other molecular contact (2). FRET is an additional nonradiative decay pathway from the excited state of a fluorophore and thus shortens its measured fluorescence lifetime.
Second, multiple mechanisms of quenching have been proposed for well-known collisional quenchers of fluorescence. For example, iodide is thought to quench via promotion of intersystem crossing (ISC) during a collision with a fluorophore (3), oxygen is thought to quench via exciplex formation (among other mechanisms) (4,5), and acrylamide has been proposed to quench via an electron transfer mechanism (6).
In summary, collision-driven mechanisms of quenching are dynamic quenching, but not all dynamic quenching is collision-mediated quenching.
Equating Static Quenching and Association between Fluorophore and Quencher
Association between a fluorophore and a quencher does not equate to a static quenching mechanism. FRET, as noted earlier, causes dynamic quenching, and FRET configurations frequently have the fluorophore and quencher associated with one another, even if indirectly through one or more molecules on which they are labels (2). Likewise, systems of fluorophore and quencher pairs that engage in photoinduced electron transfer (PET) are often intimately associated with one another (sometimes as parts of the same molecule) because of the short-range nature of this dynamic quenching mechanism (7). Conceptually, Perrin quenching, which occurs all-or-none inside or outside a sphere of action, is a static quenching mechanism that requires close proximity but not physical association (5,8). That said, Perrin quenching is a phenomenological description, and often arises in a viscous solution or solid matrix from a dynamic quenching mechanism that is very efficient but only at very short range; for example, electron transfer (9). An example of static quenching that is based on association is when a ground state fluorophore and a quencher form a non-fluorescent or weakly-fluorescent complex, as observed with some fluorophores and nucleotides (5,10).
In sum, not all static quenching requires association between quencher and fluorophore, and not all association between quencher and fluorophore equates to static quenching.
Using FRET as a Generic Term for Quenching
A surprisingly common misconception (or deduction) is that any fluorescence quenching that is neither collisional quenching nor static quenching must be FRET. The FRET terminology is not generic. Rather, it refers to a specific mechanism of energy transfer to which particular conditions and assumptions are inherent. Although there are many more nuances to FRET theory (2), the basic checklist for considering FRET as a plausible quenching mechanism is a short one. This checklist includes: a defensible expectation of an approximately 1–10 nm separation between fluorophore and quencher; overlap between the fluorophore's emission spectrum and quencher's absorbance spectrum; and a decrease in the fluorescence lifetime of the fluorophore that parallels its quenching. Note that close contact between a fluorophore and a quencher may favor other mechanisms of energy transfer (ET), such as Dexter ET.
Conferring Too Much Diagnostic Value to a Stern-Volmer Plot
A plot of F0/F versus [Q] is a Stern-Volmer plot (5). The terms F0 and F refer to the measured fluorescence intensity for a fixed amount of fluorophore in the absence and presence, respectively, of a certain concentration of quencher, [Q], with a linear trend often anticipated.
A Stern-Volmer plot is an elegant method of determining quantitative information (for example, rate constant) about fluorescence quenching when the nature of that quenching is reasonably well known; however, on its own, a single Stern-Volmer plot is unreliable for identifying how unknown quenching is mediated. The reason is that a Stern-Volmer plot may be linear or curved for multiple reasons (see Figure 1). A collisional quenching process and the formation of a non-fluorescent ground-state complex will both frequently yield straight lines, and curved plots can occur for concurrent quenching by multiple quenching processes, as well as for special cases of single processes; see references (5,8) for discussion.
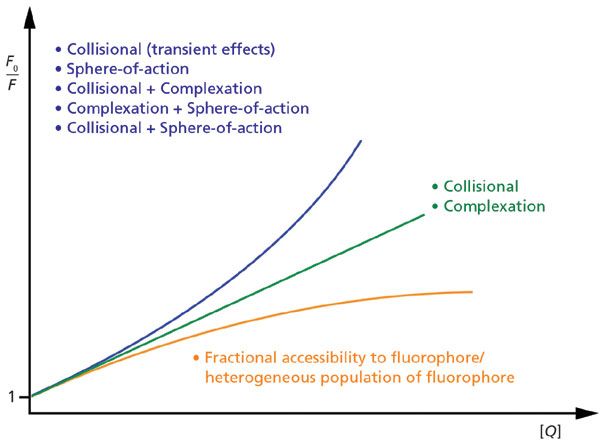
Figure 1: Diagram of generic Stern-Volmer plots based on fluorescence intensity. Linear plots, concave-up plots, and concave-down plots (to a plateau) arise from various ways in which quenching is mediated.
Given the above, the main diagnostic utility of a Stern-Volmer plot is not the qualitative trend of the plot, but rather the value of the quantitative parameter extracted from fitting the data to a quenching model. If this value is not physically reasonable, then the model of quenching likely needs to be reconsidered.
Neglecting to Rigorously Validate How Quenching Is Mediated
For the purpose of this article, mechanism refers to processes that alter the radiative or nonradiative relaxation rates of a fluorophore (for example, intersystem crossing, FRET, electron transfer, proton transfer, H-dimerization). As most quenching mechanisms have a distance dependence, mediation refers to how the necessary proximity is generated between fluorophore and quencher. For example, diffusion mediates collisional quenching, intermolecular forces mediate formation of a non-fluorescent H-dimer, and biomolecular binding interactions frequently mediate FRET (see Figure 2).
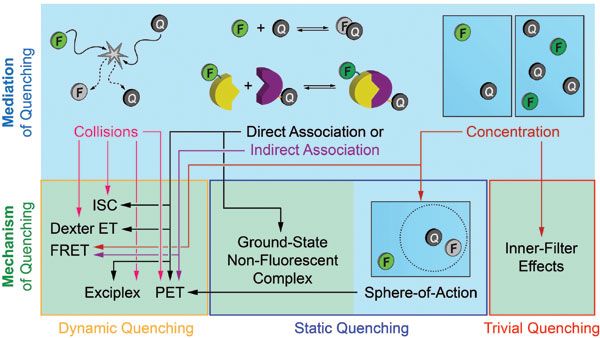
Figure 2: Flowchart that distinguishes between mediation and mechanism of fluorescence quenching, and classifies the mechanisms as dynamic, static, or trivial quenching. The flowchart is representative rather than exhaustive in its content. Note: Sphere-of-action quenching is an unusual case that mimics static quenching (no change in fluorescence lifetime) but may have an underlying dynamic quenching mechanism.
Trends in quenching efficiencies and, when applicable, the slopes or curvature of Stern-Volmer plots measured under multiple conditions, have diagnostic utility for determining how unknown fluorescence quenching is mediated. For example, changes in temperature or viscosity alter diffusion rates, changes in temperature or solvent polarity alter the value of complexation equilibrium constants, and adding competitive inhibitors, denaturants, or hydrolytic reagents can disrupt binding interactions. The opposite responses of diffusion and complexation equilibria to temperature are often cited to illustrate how these experiments can be diagnostic, but nearly any hypothesized mediation of quenching can be tested through judicious changes in conditions or additives.
The above experiments are also good guards against trivial quenching, also known as inner-filter effects. One common inner-filter effect is when the quencher reduces the excitation light available to be absorbed by the fluorophore, and another is when a quencher absorbs light emitted from the fluorophore as fluorescence (11). Progressive shifts in emission spectra that accompany increases in quencher concentration can be diagnostic of inner-filter effects (spectra generally quench uniformly for non-trivial quenching), and fluorescence lifetime measurements are often immune to inner-filter effects. Additives or changes in conditions that disrupt mediation of quenching are also diagnostic because they alter quenching efficiency without altering quencher concentration. If the expected recovery of fluorescence is not observed when the hypothesized mediation of quenching is disrupted, then either the hypothesis needs to be revised, or the quenching is actually trivial.
Undervaluing Absorbance Spectra, Excitation Spectra, and Fluorescence Lifetime Data
Emission spectra are predominantly used to measure fluorescence quenching. Useful insights can be overlooked by neglecting to also measure absorbance spectra and fluorescence excitation spectra for fluorophore-quencher mixtures. For example, dynamic quenching mechanisms do not affect the light absorption process. Absorbance spectra thus remain unchanged and useful for quantifying the concentration of fluorophores, which is both diagnostic and helpful in avoiding erroneous interpretation of inadvertent changes in fluorophore concentration as quenching. In contrast, the formation of a ground-state non-fluorescent complex between a fluorophore and a quencher may cause changes in absorbance spectra that do not propagate to excitation spectra. Shifts or changes in the shape of excitation spectra are sometimes diagnostic of an excitation-based inner-filter effect. For instances of FRET where the fluorophore of interest (donor) is quenched by another fluorophore (acceptor), the energy transfer leads to emission from the acceptor. An excitation spectrum is useful for assessing what fraction of the measured acceptor emission is from energy transfer rather than from direct excitation (the alternative is measurement of control samples with only the fluorescent acceptor).
Last, but not least, fluorescence lifetime measurements are almost imperative to deducing an unknown mechanism of quenching. There is no other direct method of establishing dynamic quenching (other parameters that depend on fluorescence lifetime, such as fluorescence anisotropy, can sometimes be substituted as indirect measurements). Discrepancies between lifetime- and intensity-derived quenching efficiencies or Stern-Volmer plots are thus diagnostic of static mechanisms of quenching, or mixed static and dynamic mechanisms, or an inner filter effect alongside quenching.
Conclusion
In summary, a robust and meaningful analysis, interpretation, and discussion of fluorescence quenching is developed through multiple sets of measurements, attention to both the mechanism and mediation of quenching, precise use of terminology, and knowledge of the hallmarks and nuances of different quenching behaviors.
References
(1) S.E. Braslavsky, Pure Appl. Chem. 79, 293–465 (2007).
(2) N. Hildebrandt, C.M. Spillmann, W.R. Algar, T. Pons, M.H. Stewart, E. Oh, K. Susumu, S.A. Díaz, J.B. Delehanty, and I.L. Medintz, Chem. Rev. 117, 536–711 (2017).
(3) A. Chmyrov, T. Sandén, and J. Widengren, J. Phys. Chem. B 114, 11282–11291 (2010).
(4) K. Kikuchi, C. Sato, M. Watabe, H. Ikeda, Y. Takahashi, and T. Miyashi, J. Am. Chem. Soc. 115, 5180–5184 (1993).
(5) J.R. Lakowicz, Principles of Fluorescence Spectroscopy, 3rd ed. (Springer US, New York, New York, 2006).
(6) M.R. Eftink, T.J. Selva, and Z. Wasylewski, Photochem. Photobiol. 46, 23–30 (1987).
(7) S. Doose, H. Neuweiler, and M. Sauer, Chem. Phys. Chem. 10, 1389–1398 (2009).
(8) B. Valeur, Molecular Fluorescence: Principles and Applications (Wiley-VCH Verlag GmbH, Weinheim, 2001).
(9) S. Murata, M. Tachiya, Chem. Phys. Lett. 194, 347–350 (1992).
(10) D.A. Dunn, V.H. Lin, and I.E. Kochevar, Photochem. Photobiol. 53, 47–56 (1991).
(11) T. Wang, L-H. Zeng, and D-L. Li, Appl. Spectrosc. Rev. 52, 883–908 (2017).
W. Russ Algar and Melissa Massey are with the Department of Chemistry at the University of British Columbia, in British Columbia, Canada. Direct correspondence to: algar@chem.ubc.ca

FT-IR Microscopy, Part 2: Mid-IR Sampling with DRIFTS, IRRAS, and ATR
February 14th 2025Fourier transform infrared (FT-IR) microscopy using reflection methods (diffuse reflection, reflection/reflection-absorption, or attenuated total reflectance) typically requires less sample preparation than transmission. However, optimal results will depend upon the sample and, in particular, the sample surface.
Key Points to Remember When Using Internal Standards for Sample Analysis by ICP-OES
August 2nd 2023Using internal standards is a common technique to correct for variations in sample matrices and the effect this has on analyte intensities. There are several basic criteria to be considered when using internal standards: selection of appropriate internal standards, the concentration added to the solutions analyzed, setting up in the correct view (axial vs. radial), how to introduce the internal standard to the solutions to be analyzed, and evaluating the resulting data. Each of these topics are considered and suggestions presented.
A Brief Look at Optical Diffuse Reflection (ODR) Spectroscopy
August 1st 2023In this short overview, we consider cases for diffuse reflection spectroscopy and introduce the Kubelka-Munk diffuse reflectance formula. We conclude by comparing diffuse transmittance, diffuse reflectance, logarithmic transforms of both, and the Kubelka-Munk transform for mid-infrared spectroscopy of the same sample.
Key Steps to Follow in a FRET Experiment
August 1st 2022Förster resonance energy transfer (FRET) is a versatile part of the toolbox of fluorescence methods. This through-space, photon-less energy transfer process between a donor fluorophore and an acceptor chromophore is perhaps most famous for its utility as a “molecular ruler” that can resolve nanometer-scale distances. FRET is also a popular and advantageous basis for biomolecular assays and sensors.