Laser Ablation and Inductively Coupled Plasma–Time-of-Flight Mass Spectrometry—A Powerful Combination for High-Speed Multielemental Imaging on the Micrometer Scale
Spectroscopy
Over the last few decades, elemental imaging using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) has emerged as an important tool in the study of solid samples from a variety of scientific disciplines, including medicine, biology, and geology. This article highlights recent analytical trends towards high-speed, high-spatial resolution, multi-elemental imaging that became possible with advances in both LA and ICP-MS technology, including the design of fast-washout ablation cells and commercialization of high-speed ICP-MS such as time-of-flight mass analyzers (TOFMS), This study will demonstrate the new imaging approach by coupling LA with an-ICP-TOFMS system (icpTOF from TOFWERK, Thun, Switzerland) on two application areas: quantitative mapping of trace elements in a sulfide mineral (sphalerite), and imaging of the distribution of a chemotherapy drug (Cisplatin) in a rat kidney. High-performance LA-ICP-TOFMS provides researchers with an effective new tool to study biological and geological processes, with much greater speed and in much greater detail than previously possible with conventional ICP-MS instrumental designs.
Over the last few decades, elemental imaging using laser-ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS) has emerged as an important tool in the study of solid samples from a variety of scientific disciplines, including medicine, biology, and geology. This installment of “Atomic Perspectives” highlights recent analytical trends toward high-speed, high-spatial resolution, multielemental imaging that became possible with advances in both LA and ICP-MS technology, including fast-washout ablation cells and time-of-flight mass spectrometry (TOF-MS) analyzers. This study demonstrates the new imaging approach by coupling LA with ICP-TOF-MS in two application areas: quantitative mapping of trace elements in a sulfide mineral (sphalerite) and imaging of the distribution of a chemotherapy drug (cisplatin) in a rat kidney. LA-ICP-TOF-MS provides researchers with an effective new tool to study biological and geological processes, with greater speed and in greater detail than previously possible with conventional ICP-MS instruments.
Since its first appearance in the mid-1980s (1), laser-ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS) has established itself as a routine method for the quantitation of trace elements in solid samples (2). Recently, LA-ICP-MS has further become an important tool for elemental imaging applied in geological, biological, and medical research studies. To date, the most common imaging approach is to run the laser in continuous-scan mode, which involves firing the laser continuously with a specific repetition rate (usually 1–10 Hz) while slowly moving the stage with the mounted sample underneath the laser beam. The image is then constructed by ablating parallel lines on the sample surface. The ablated aerosol is washed out of the air-tight ablation chamber (cell) to the ICP with a continuous flow of inert gas (for example, He).
The washout times for conventional ablation cells are on the order of 0.5–30 s (3). After ionization in the ICP, the ablation signal is measured in a time-resolved manner, most commonly using a quadrupole or a sector field mass spectrometer. These instruments operate sequentially, resulting in the measurement of only one isotope at a time. For the analysis of multiple isotopes or elements, sequential peak-hopping must be performed which, depending on the number of elements of interest, can become very restrictive and time-consuming (4). The conventional approach to laser-ablation imaging is associated with drawbacks in spatial resolution and speed of analysis, which is usually limited to 1–2 pixels/s (3). The recent advent of fast-washout ablation cells and high-speed ICP-TOF-MS allowed some of these limitations to be overcome by enabling spot-resolved imaging.
Technological Advances in Laser-Ablation Cells
Recently, the design of laser-ablation cells has evolved toward ever-faster washout times to reduce the duration of single LA signals (3). Faster washout is most readily achieved by reducing the internal volume of the cell and the tubing that transports the aerosol to the ICP (5,6). In addition, maintaining laminar gas flows during aerosol transport and the use of two-volume cells can reduce washout times. Commercially available designs include the TwoVol2 cell with a dual concentric injector (DCI) (Electro Scientific Industries), and the HelEx II cell with an aerosol rapid introduction system (ARIS) (Teledyne CETAC Technologies). The washout times of these next-generation ablation cells are on the order of tens of milliseconds.
There are many advantages of fast-washout cells, which are mainly related to the effect of reduced aerosol dispersion. This effect implies that the ablated material from an individual laser pulse arrives at the ICP-MS in a narrowly focused peak (over time) as opposed to a slowly decaying peak. The arrival of sharp ablation peaks minimizes pulse-to-pulse intermixing, which allows for better-resolved detection of single LA events by the ICP-MS system, and makes it possible to fire the laser at higher repetition rates (up to 100 Hz). The narrower ablation signals are also more concentrated over time-that is, they have higher peaks-leading to improved signal-to-noise ratios in the ICP-MS system. The possibility of using smaller laser spots with higher signal-to-noise ratios enables the improvement of spatial resolution. Finally, fast washout allows for faster spot-resolved LA-ICP-MS imaging, thereby greatly reducing the overall time of analysis.
Time-of-Flight MS
To capitalize on the benefits of fast-washout ablation cells, a mass spectrometer capable of extremely fast and simultaneous multielement data acquisition is required, and in particular it must be able to handle rapid transient signals from these cells. The fundamental principles of modern time-of-flight MS systems are exemplified in Figure 1 (7). Here, we focus on the key capabilities that make the technique capable of handling the short transient signals from fast-washout laser-ablation cells.
Figure 1: (a) Schematic diagram of the time-of-flight (TOF) mass analyzer. The primary ion beam travels from the ICP interface (blue frame) into the extraction zone of the TOF mass analyzer (yellow frame). From there, ions are accelerated orthogonally to the beam direction into the field-free flight tube at a frequency of ~33 kHz. Gaining equal energy from this acceleration, different ions travel at different speeds directly related to their mass-to-charge ratio (m/q), with lighter ions traveling faster than heavier ions. At the detector, the intensity and time-of-flight of ions from the entire mass spectrum are recorded. (b) Measured time-dependent signal. The top diagram shows the measured intensity (in mV) of different ions against their recorded time of flight. By performing a mass calibration to known analyte peaks in the spectrum, the time-of-flight values are transformed into mass-to-charge ratios (in Thomson [Th] units).
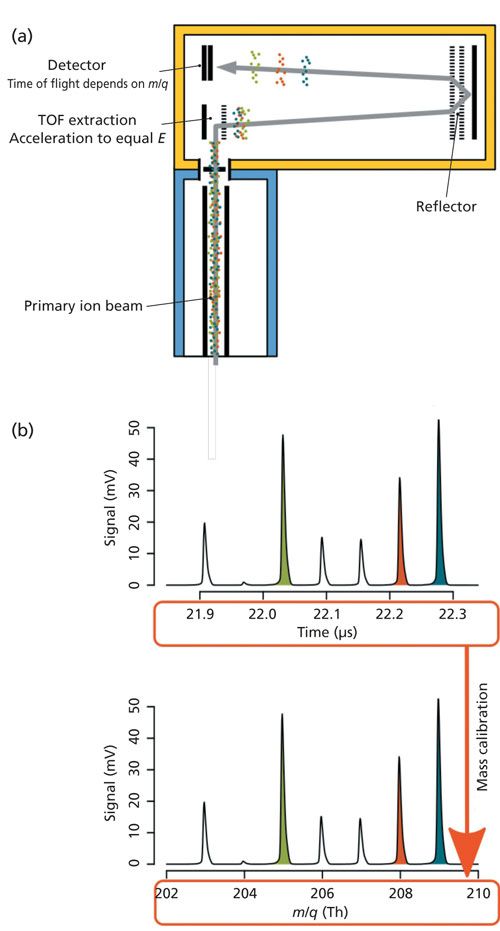
ICP-TOF-MS is well-suited for the shot-resolved imaging approach because of its simultaneous and fast multielement analysis. In TOF-MS, the entire mass spectrum is measured simultaneously with each ion package extraction, as shown in Figure 1. Different isotopes from this package are then separated based on their time of flight in traversing the region from extraction to detector, which is directly related to their mass-to-charge ratio. Thus, in contrast to conventional sequential mass spectrometers, it is not necessary to predefine or limit a range of isotopes for analysis. Moreover, an ICP-TOF-MS system can record complete mass spectra at a rate of 30 µs, which amounts to a maximum of ~33,000 spectra/s This capability allows short transient signals from single laser pulses to be temporally resolved with sufficient sampling density (for example, up to ~1000 spectra per 30 ms pulse). In practice, however, multiple spectra are integrated to improve counting statistics and to simplify data processing. Because of this simultaneous measurement of complete mass spectra, TOF-MS systems can have a higher total ion utilization than sequential mass spectrometers, and as a result, a greater proportion of the ablated sample is actually used for analysis.
Analytical Requirements for LA-ICP-TOF-MS Imaging
The fundamentals of LA-ICP-MS imaging experiments are well described in the public domain (3). Here, we discuss some of the technical requirements for high-speed, high-spatial resolution, multielemental imaging using LA-ICP-TOF-MS.
One major requirement for spot-resolved imaging is a high level of synchronization between the LA system and the ICP-TOF-MS system to confidently assign every laser shot signal to the correct pixel in the image. Until now, laser-ablation imaging has been performed mostly in a nonsynchronized manner and integration intervals were defined based on analyte signal intensities using different data post-processing algorithms. This approach, however, does not work for all samples because in some cases the resulting signal may be too low, or too inconsistent, to properly define integration intervals. Thus, for routine high-performance imaging, a synchronized approach is preferred.
To realize spot-resolved single-pixel synchronization, the laser system needs to send an electronic trigger pulse (via a transistor-transistor logic [TTL] trigger cable) with each laser shot to start data acquisition at the instrument. Here, the travel time of the ablated aerosol from the cell to the detector must be taken into account. This delay is sample-specific, as a result of different ablation characteristics of different materials, and it also depends on the gas conditions in the cell. Therefore, the delay has to be calibrated before analysis. Accounting for these different factors online-that is, during the method development stage rather than in post-processing-greatly simplifies and speeds up the construction of images. Ultimately, this approach enables images to be displayed in real-time, thereby providing users with a higher level of control over the imaging experiment. This process is shown in Figure 2.
Figure 2: Schematic of the principle of precisely triggered laser ablation based on communication between the laser computer and ICP-TOF-MS instrument. With every single laser pulse, a trigger is sent to the instrument to start the data acquisition. The delay time between ablation and detection depends on the sample material and the conditions within the ablation cell and has to be calibrated prior to analysis.
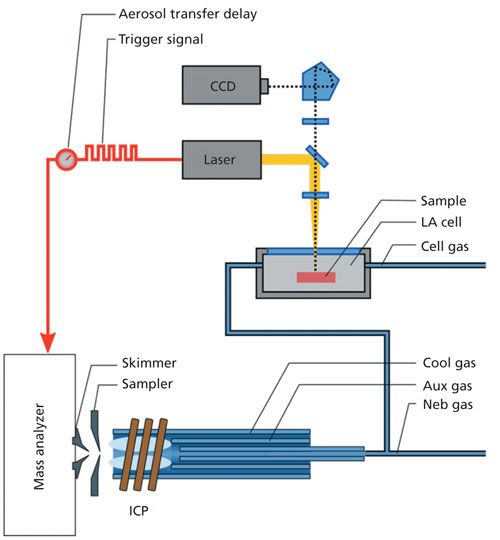
In principle, the described precise triggering for shot-resolved imaging can be performed with any LA system that allows for sending such trigger pulses. So far, this approach has been tested with the ICP-TOF-MS system coupled to commercial LA systems from ESI (NWR213) and Teledyne CETAC Technologies (Analyte G2 excimer laser). The maximum spatial resolution is defined by the minimum laser spot size. It’s worth pointing out that modern LA systems typically offer spot sizes down to 1 µm. Another prerequisite for high-resolution imaging is that the sample stage can be precisely controlled, which for most LA systems is on the order of <1 µm.
Application Examples for LA-ICP-TOF-MS Imaging
In our investigations, the applicability of LA-ICP-TOF-MS imaging is demonstrated on the basis of a geological and a biological case study using an icpTOF ICP-TOF-MS system (TOFWERK). The instrument has been described in the open literature, but for more details about its performance and specifications, the reader is advised to refer to the following citations (4,8).
Geological Case Study
The geological example is a polished thin section of the mineral sphalerite (ZnS) displaying distinct internal growth zoning (Figure 3, top left). The sample has been thoroughly characterized by conventional LA-ICP-MS in a study by Belissont and colleagues (9). To evaluate the distribution of trace elements in the various zones, which is important for deciphering the underlying mineralization processes, the authors analyzed 135 single spots with laser spot sizes ranging from 32 to 60 µm. However, because zoning features may be much smaller and large single-spot analyses may not be representative, laser-ablation imaging at high spatial resolution was preferred.
Figure 3: Geological application example of high-speed, high-spatial resolution, multielemental imaging by LA-ICP-TOF-MS. Top left: Optical microscope image of a sphalerite (ZnS) grain with distinct growth zoning. A single ablation line is indicated by the horizontal white line with the corresponding signal for one isotope (121Sb) shown below. Top right: Time-resolved signal for one isotope (121Sb) resulting from a single laser shot which was integrated to obtain one pixel in the elemental image below. The corresponding position of the single pixel in the line scan and in the final image are indicated by the white arrows. Bottom: Processed trace element images (color-coded by concentration) recorded with a spatial resolution of 5 µm within a total analysis time of ~80 min. Groups of elements with similar distribution patterns in sphalerite are indicated by blue, green, and yellow frames.
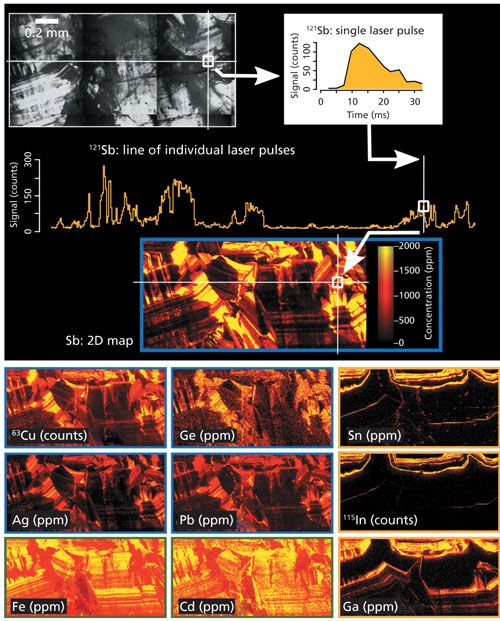
Multielemental imaging by LA-ICP-TOF-MS was performed by using an ESI NWR213 laser-ablation system, equipped with a Bloodhound low aerosol dispersion system (an upgrade to the TwoVol2 cell featuring a small-volume ablation cup and a dual concentric injector), coupled to an icpTOF ICP-TOF-MS system. On the sphalerite section, an area of 1 mm by 2 mm was imaged at a spatial resolution of 5 µm (laser spot size). Ablation was performed at a 20-Hz laser repetition rate while moving the stage at a speed of 100 µm/s. Each laser pulse produced a ~30-ms transient signal, and adjacent pulses could easily be resolved because of the high acquisition speed of the ICP-TOF-MS system. To reliably link each laser-ablation event to a detected signal, the ICP-TOF-MS acquisition was synchronized with the pulsing of the laser, so that the signal from a single laser pulse would be integrated into a single pixel in the image. A representative transient signal from a single laser pulse used for the integration of a single pixel is shown in Figure 3, top right (only the signal for 121Sb is shown).
The total analysis time of this multielemental image was ~80 min, with a complete integrated mass spectrum recorded for each 5-µm pixel. The intensity images were quantified against the MASS-1 sulfide standard to obtain elemental concentrations in parts per million. The resulting high-resolution elemental images clearly reveal distinct distribution patterns for different elements, which allow for sorting them into groups (as indicated by blue, green, and yellow frames in the bottom of Figure 3). The correlation of certain trace elements is a function of their similar geochemical behavior in sphalerite. Such trace-element trends can be used to discriminate between different genetic types of mineralization (9).
Biological Case Study
The biological example is a thin section of a rat kidney that had been perfused with cisplatin, a Pt-based anti-tumor agent (10). Although such compounds are frequently used in chemotherapy, their exact distribution, accumulation, and metabolism in organs remain poorly understood. Laser ablation imaging can shed new light on these unknowns and help to optimize Pt-based treatments and minimize side-effects.
Quantitative multielemental imaging by LA-ICP-TOF-MS was performed using a CETAC Analyte G2 excimer laser equipped with a HelEx II cell and an ARIS device. Ablation was performed on an area of 10 mm x 7 mm, using a 100-Hz laser repetition rate with a square 20-μm laser spot, while moving the stage at a speed of 200 μm/s. At this scan speed, 10 laser pulses were delivered to every 20-μm spot and 10 signals of single laser pulses were integrated to obtain the signal of one pixel. This increased dosage (laser pulses per pixel) was necessary to ablate through the entire sample section, which was required for quantification with custom-prepared external standards. The images were processed to obtain elemental concentrations using the Iolite data processing software package (School of Earth Sciences, University of Melbourne, Australia) (11). The total acquisition time of the entire multielemental image (70 mm2) was about 5 h. With the conventional approach of LA-ICP-MS imaging it can take up to 30 h to image a 5-mm2 area with a spatial resolution of 15 μm (12).
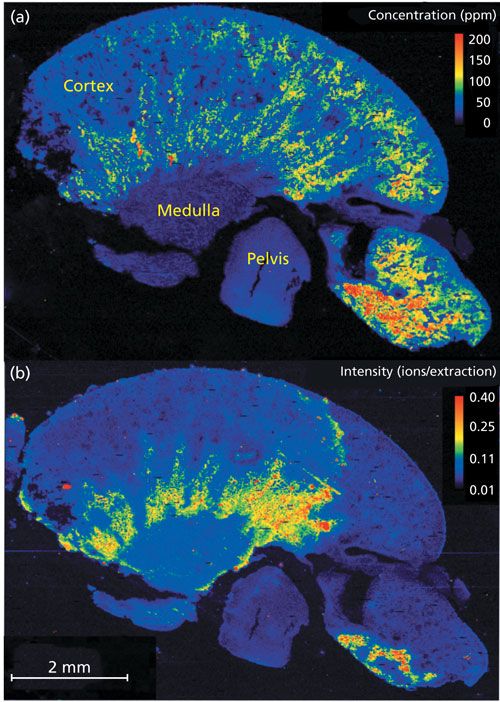
Higher Pt concentrations were detected in the cortex region relative to the medulla and pelvis (Figure 4a). Other trace metals, such as Cu (Figure 4b), were detected in the sample and might provide additional information on cisplatin uptake and distribution.
Figure 4: Biological or medical application example for high-speed, high-spatial resolution, multielemental imaging by LA-ICP-TOF-MS performed on a section of a rat kidney perfused with the chemotherapy drug cisplatin. The elemental images were recorded at a spatial resolution of 20 µm within a total analysis time of ~5 h. (a) Concentration of Pt in ppm. (b) Intensity distribution of Cu in counts per extraction.
Conclusions
Because of the recent technological advances in laser-ablation cell design and time-of-flight mass spectrometry, the method of LA-ICP-TOF-MS for high-speed, high-spatial resolution, multielemental imaging is poised to become an important analytical tool for researchers from various scientific disciplines. The recent progress will be further exploited by the development of high-performance data reduction software to support spot-resolved quantification strategies. Automated data processing using pixel-by-pixel quantification will enable the location of regions of interest within an imaged sample, based on specified isotopic or elemental compositions. For example, this methodology will simplify the search for accessory phases (low modal abundances) within rock samples, or tumor-affected tissue within biological samples. Moreover, progress is currently being made toward real-time display of multielemental images to overcome the limitation of imaging experiments being recorded “blindly.” Presently, the images only become visible after elaborate data post-processing. Real-time imaging will greatly reduce the risk of losing valuable analytical time or precious sample material.
Acknowledgments
We would like to acknowledge our collaborators Rémi Belissont and Marie-Christine Boiron at the Université de Lorraine and Oliver Bolle Bauer, Michael Sperling, and Uwe Karst at the University of Münster for providing sample material. Leif Summerfield (ESI) is thanked for technological development and support. ESI and Teledyne CETAC Technologies are thanked for their professional assistance with their laser-ablation systems. We thank the Iolite team for continued support with post-processing of icpTOF data. Finally, special thanks to Robert Thomas for the invitation to contribute to his “Atomic Perspectives Column” in Spectroscopy.
References
- A.L. Gray, Analyst110(5), 551–556 (1985).
- D. Günther and B. Hattendorf, TrAC, Trends Anal. Chem. 24(3), 255–265 (2005).
- A. Gundlach-Graham and D. Günther, Anal. Bioanal. Chem. 408(11), 2687–2695 (2016).
- M. Wiedenbeck, Elements12(5), 370–372 (2016).
- H.A. Wang, D. Grolimund, C. Giesen, C.N. Borca, J.R. Shaw-Stewart, B. Bodenmiller, and D. Günther, Anal. Chem. 85(21), 10107–10116 (2013).
- S.J. Van Malderen, A.J. Managh, B.L. Sharp, and F. Vanhaecke, J. Anal. At. Spectrom.31(2), 423–439 (2016).
- M. Guilhaus, D. Selby, and V. Mlynski, Mass Spectrom. Rev. 19(2), 65–107 (2000).
- L. Hendriks, A. Gundlach-Graham, B. Hattendorf, and D. Günther, J. Anal. At. Spectrom.32(3), 548–561 (2017).
- R. Belissont, M.C. Boiron, B. Luais, and M. Cathelineau, Geochim. Cosmochim. Acta126, 518–540 (2014).
- O.B. Bauer, C. Köppen, O. Hachmöller, G. Ciarimboli, H. Schurek, M. Sperling, O. Borovinskaya, and U. Karst, “Novel Platinum Bioimaging Approaches Using LA-ICP-ToF-MS and On-Line Isotopic Dilution Analysis,” presented at ANAKON, Tübingen, Germany, 2017.
- C. Paton, J. Hellstrom, B. Paul, J. Woodhead, and J. Hergt, J. Anal. At. Spectrom.26(12), 2508–2518 (2011).
- J. Lear, D. Hare, P. Adlard, D. Finkelstein, and P. Doble, J. Anal. At. Spectrom. 27(1), 159–164 (2012).

Yannick Bussweiler holds a PhD in geology from the University of Alberta, Canada. During his research on the geochemistry of different mineral components from Earth’s mantle he has gathered profound applied experience in LA-ICP-MS. In 2017, he joined TOFWERK AG in Thun, Switzerland, as an application scientist at specializing in new applications that combine laser ablation with time-of-flight mass spectrometry.

Olga Borovinskaya is an application scientist at TOFWERK AG working on the development of new application fields for time-of-flight based ICP-MS. She completed her PhD in the group of Trace Element & Micro Analysis at ETH Zurich, Switzerland. Her expertise lies in single-nanoparticle analysis and high-resolution, high-speed LA-ICP-MS applications.

Martin Tanner holds a PhD in analytical chemistry from ETH Zurich, Switzerland. During his doctoral research he combined an ICP source with a time-of-flight mass analyzer from TOFWERK for the detection of short transient signals. As project manager at TOFWERK he has led the development and is now responsible for the icpTOF, a commercial instrument which adds the versatility of TOF-MS technology to the iCAP RQ from Thermo Scientific.

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.