Meeting Requirements of the EPA Contract Laboratory Program ILM05.3 Using Inductively Coupled Plasma-Mass Spectrometry: An Update
This report demonstrates that it is possible to meet and exceed EPA "statement of work" requirements using ICP-MS.

The United States Environmental Protection Agency (EPA) was formed in 1970 in order to cope with increasing public demand to improve environmental quality. Among its numerous activities is the monitoring of contaminated and potentially contaminated sites within the Superfund Program, created under the 1980 Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), and currently under the 1986 Superfund Amendments and Reauthorization Act (SARA). In order to achieve these tasks, the EPA Office of Superfund Remediation and Technology Innovation (OSRTI) employ contract laboratories within their Contract Laboratory Program (CLP) to provide analytical services by following statements of work (SOWs) that include comprehensive and prescriptive analytical methodologies.
The inorganic SOW, ILM05.2, released in 2001, provided methodological details on the determination of various parameters including inorganic analytes using techniques such as atomic absorption spectrophotometry (AAS), inductively coupled plasma atomic emission spectrometry (ICP-AES), and inductively coupled plasma mass spectrometry (ICP-MS). Recently, this SOW was superseded by ILM05.3, which clarified and streamlined several sections of the methodology.
ILM05.3 is the inorganic SOW that defines the analytical procedure for determination of metal contaminants in water, soil, sludge, sediments, and various solid wastes found at potentially hazardous contaminated or suspected contaminated sites. Samples are digested and the solutions analyzed for 16 analytes on the ICP-MS target analyte list (TAL): antimony, arsenic, barium, beryllium, cadmium, chromium, cobalt, copper, lead, manganese, nickel, selenium, silver, thallium, vanadium, and zinc.
In this article, we will demonstrate that ICP-MS is a viable method for complying with the requirements of CLP ILM05.3. The X-Series is a versatile instrument for the analysis of the wide variety of complex waste samples detailed in ILM05.3. These waste samples represent vastly differing matrices, with different levels of total dissolved solids and thus, a wide range of potential spectroscopic and nonspectroscopic matrix-induced interferences. Despite these variations, the conventional analytical figures of merit that are detailed in the SOW will be used to show that ICP-quadrupole MS is an excellent tool for the successful analysis of the target analytes. It is worthwhile to mention that the implementation of these compliant routines also is important for non-CLP labs that require CLP-like services to their customers.
QA/QC Requirements
ILM05.3 (2) is quality control (QC) intensive, requiring the user to establish and register comprehensive figures of merit for stringent validation of sample data. The laboratory must demonstrate these figures of merit as a measure of their analytical capability. These include periodic analysis of laboratory reagent blanks, fortified blanks, and calibration verification, quality control samples for evaluating matrix interferences. Quality control requirements are summarized in Table I. A summary of changes in ILM05.3 follows.
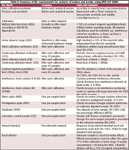
Table I. Summary of QC requirements for analysis of waters and wastes using EPA CLP ILM0.
Target analyte list (TAL). Aluminum, previously listed in ILM05.2, has been excluded from the ILM05.3 TAL. Aluminum now is analyzed by ICP-AES because of the potential for high concentrations in waste samples.
Contract required quantitation limits (CRQLs). The change in the CRQLs has an impact on several QC checks based upon these CRQLs; for example, CRI, ICSA, and blank checks (ICB, CCB).
Sample dilution. Sample solutions containing analyte concentrations exceeding the LDR must be diluted. The criterion for sample dilution has been changed to state that the concentration in the diluted solution must be within the upper 75% of the calibrated or linear range. This is to ensure the quality of data obtained from the dilution is not reduced by over-dilution.
Sample preparation. Several practical changes have been made in sample preparation methodology. ILM05.3 allows the use of polymeric beakers and watch glasses for digestion to minimize contamination. Fluoropolymers in particular reduce the likelihood of contamination due to their hydrophobic surface properties.
The previous ILM05.2 specified ultrapurity acids for preparation of sample and standard solutions. In ILM05.3, this requirement is excluded, because the cost of ultrapurity grade reagents can be prohibitively high for routine analytical work and analyte concentrations generally are high enough so that reagent grades are sufficient for determinations.
For "direct analysis" in ILM05.2, samples only were acidified and filtered or centrifuged before analysis (preparation code NP1). In ILM05.3, this option has been excluded, and a preparation option is used (HW3), essentially consisting of heating a 100-mL sample (93–95 °C) with 1 mL of nitric acid and 1 mL of hydrochloric acid in a covered vessel until the volume is reduced by half. The solution then is refluxed for a further 30 min, cooled, filtered, and made to a final volume of 100 mL.
Resolution. Within the "Tune" analysis, ILM05.2 specified a resolution check, whereby the peak width at 5% peak height must be < 0.75 amu. In ILM05.3, this requirement has been changed to allow the resolution to be checked according to the instrument manufacturers' recommendations.
Internal standards. The minimum number of internal standards has been increased from three to five spanning the mass-range of interest. ILM05.3 lists the following possibilities: 6 Li+ , 45 Sc+ , 89 Y+ , 103 Rh+ , 115 In+ , 165 Ho+ , 159 Tb+ , 175 Lu+ , and 209 Bi+ . In this application we used 6 Li+ , 45 Sc+ , 115 In+ , 159 Tb+ , and 209 Bi+ . Instructions also have been clarified to state that the "Tune" solution should not be analyzed with internal standards and that the internal standard element concentrations be consistent throughout the run.
When internal standard recoveries exceed the permissible range for serial dilution samples, the actions are simplified. Because the normal action is to perform a dilution, this now is performed without the additional analysis of the blank, which facilitates automated analytical runs.
Analysis sequence. In ILM05.3, the sequence of analysis of QC samples and solutions has been clarified to indicate the proper analysis order, which was difficult to interpret in ILM05.2. In ILM05.3, the sequence is: Tune solution, Calibration Blank, Calibration Standard(s), ICV (initial calibration verification), ICB (initial calibration blank), CRI at the CRQL, ICSA, ICSAB, CCV (continuing calibration verification),CCB (continuing calibration blank), 10 samples, CCV, CCB, seven samples, CRI, CCV, CCB, 10 samples, etc.
Instrumentation
In a previous report (1), it was shown that the X-Series ICP-MS (Thermo Electron, Waltham, MA) achieved requirements of CLP ILM05.2. Here, an X-Series ICP-MS was used for the performance evaluation within the requirements of ILM05.3. An Environmental Interface (Xi sampler and skimmer cones, Thermo Electron, Application Note AN-E0620, 2002) was used in order to provide reduced matrix tolerance and enable simultaneous determination of both matrix and trace analytes. Optimized instrument conditions are listed in Table II.

Table II. Typical operating conditions for analysis of liquid and solid wastes using the X-Series ICP-MS and EPA CLP ILM05.3.
Reagents. Tuning solutions containing 10 μg/L of Be, Mg, Co, In, Ba, Ce, Tl, Pb and Bi, were used for optimizing ICP operating conditions, plasma position, and ion lens settings. Commercially available multielement stock solutions (EPA Productivity Pack Solutions, Thermo Electron Corp.) were diluted stepwise for calibration, validation and spiking. Ultrapure water (>18MΩ cm Milli-Q) and nitric acid (Superpure, Romil, Cambridge, UK) were used to make all solutions.
Calibration. The elements and isotopes used are listed in Table III. The concentrations of the trace-element calibration standards were 250–500 μg/L (Be, V, Cr, Mn, Co, Ni, Cu, Zn, As, Se, Ag, Cd, Sb, Ba, Tl, Pb). Internal standards, 10 μg/L of Li, Sc, Y, In, Tb, and Bi prepared from a stock solution containing 1 mg/L in 1% (v/v) nitric acid were added online via a mixing tee.
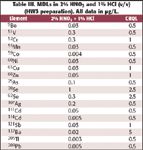
Table III. MDLs in 2% HNO3 and 1% HCl (v/v) (HW3 preparation). All data in μg/L.
Data Processing. The CLP SOW ILM05.3 requires that the data meet certain specifications and that the output is registered. This is ensured using the integral QA/QC software provided with the X-Series ICP-MS.
Results
CRQL. Table III lists the MDL values from the X-Series ICP-MS in the HW3 matrix (2% v/v nitric acid, 1% v/v hydrochloric acid). The data indicate that the requirement of 50% of the CRQLs was achieved easily, in most cases by one order of magnitude.
Daily performance using a tune sample. Analysis commenced after a minimum warm-up time of 30 min. Daily performance checks, which included mass calibration verifications, sensitivity, % CeO/Ce, and short-term precision tests, were made using a 10-μg/L tune solution.

Figure 1. Performance report detailing the results of the mass calibration of a tuning solution. The values in the top box in each graph indicates the error (amu) associated with peak calibration, the value in the bottom box indicates peak width (amu) at 5% peak height. (a) Mass calibration, peak width, and peak resolution. (b) Summary of maximum, minimum, and peak widths.
Figures 1 and 2 show compliant results for a tune solution, including the peak resolution check as specified in ILM05.2. Data were collected using the automated, custom defined performance check sequence within the X-Series ICP-MS PlasmaLab software. In Figure 1, the values in the boxes indicate the error (amu) associated with peak calibration, and the values in the bottom box indicate peak width (amu) at 5% peak height. Typical short-term % RSDs of <0.5% obtained for five acquisitions, instrument background, less than 0.2 cps, and oxide levels (156 CeO+ /140 Ce+ ) less than 2%, were satisfactory.
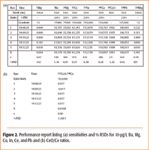
Figure 2. Performance report listing (a) sensitivities and % RSDs for 10-μg/L Ba, Mg, Co, In, Ce, and Pb and (b) CeO/Ce ratios.
Calibration verification. A continuing multielement calibration verification (CCV) solution was used to validate calibration accuracy (test for linearity and drift) immediately after calibration. The solution was prepared from calibration stock solutions and was aspirated every 10 samples. The initial calibration verification (ICV) solution was prepared from a second source and was used for independently checking stability of calibration standards and calibration drift. ICV and CCV data listed in Table IV are well within the 10% specification. CRI values confirm that accuracy at the CRQL is well within 10% of the values listed.

Table IV. Results for QC samples ICV, CRI, and CCV. Recoveries for ICV and CCV are within the 90â110% requirement of ILM05.3. All data in μg/L.
Interference checks. Interference check solutions (ICSA and ICSAB) were used to check for spectroscopic (polyatomic ions) and nonspectroscopic interferences (physical effects that occur in the sample introduction system, in the plasma and in the plasma-MS interface).
Data for the ICSA solution must be less than three times the CRQL value for each target analyte, while the measured values for the ICSAB solution must be within 20% of the known value. Table V shows that the measured values are well within the allowed ranges and therefore the analysis is sufficiently free of interferences.
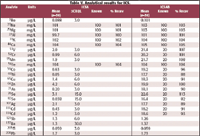
Table V. Analytical results for ICS.
QC samples. At least one unknown sample per batch must be reanalyzed as a duplicate, after serial dilution and after spiking with target analytes for recovery determination. Spike recoveries must be within 75–125%. Table VI lists the results from these tests with borehole water. All requirements are met.
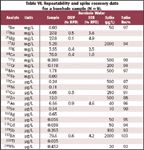
Table VI. Repeatability and spike recovery data for a borehole sample (N = 9).
Conclusion
EPA has made several practical updates to the CLP SOW for ICP-MS in ILM05.3 for routine analysis of effluents and solid wastes. The data in this report demonstrate that it is possible to meet and exceed the requirements of the SOW using ICP-MS. MDLs in the nanogram-per-liter range are achieved routinely for the majority of analytes, even in the HW3 matrix. Stable and accurate results are achieved consistently for all QC samples, and the satisfactory results of ICS solutions demonstrate the freedom from interference.
References
1. Thermo Electron Corporation, US EPA Method ILM05.2D Using the X Series ICP-MS, App. Note AN-E0620, http://www.thermo.com/elemental.
2. ILM05.3 can be downloaded free of charge from the following website: http://www.epa.gov/superfund/programs/clp/ilm5.htm.
Bill Spence and Andrew Walder are with Thermo Electron Corporation, ICP-MS Facility (Winsford, Cheshire, U.K.).
Isaac Brenner is with the Environmental Analytical Laboratory (Jerusalem, Israel).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.