Microwave Digestion for Elemental Impurities Analysis According to ICH and USP Guidelines
Spectroscopy
In this study, recovery rates between 92% and 105%, combined with very low variations (RSD
Because of landmark regulatory changes implemented by the United States Pharmacopeia (USP) and the International Conference on Harmonization (ICH), the determination of elemental impurities in drug products will be mandatory by January 1, 2018. Elemental impurities pose not only a toxicological risk to the patient, but may also affect the quality and efficacy of drug products. The updated ICH and USP guidelines incorporate the use of modernized sample preparation and analytical techniques such as inductively coupled plasma–mass spectrometry (ICP-MS). In this article, we demonstrate the suitability of a microwave reaction system to digest samples with varying and complex compositions, such as enteric-coated tablets, syrups with high sugar content, and fish oil–based nutritional supplements.
Because of landmark regulatory changes implemented by the United States Pharmacopeia (USP) and the International Conference on Harmonization (ICH), the determination of elemental impurities in drug products will be mandatory by January 1, 2018. Elemental impurities pose not only a toxicological risk to the patient, but may also affect the quality and efficacy of drug products.
For more than 100 years, USP <231> has been the standard method for the determination of elemental impurities in drug products and raw materials. This method suffers from several known limitations, including labor-intensive sample preparation and under-recoveries of key elements such as As, Cd, and Hg. The updated ICH and USP guidelines incorporate the use of modernized sample preparation and analytical techniques such as inductively coupled plasma-mass spectrometry (ICP-MS).
Microwave digestion is a safe, efficient, and reproducible sample preparation technique for a wide range of drugs before analysis by ICP-MS. In this article, we demonstrate the suitability of a microwave-assisted closed-vessel reaction approach to digest samples with varying and complex compositions, such as enteric-coated tablets, syrups with high sugar content, and fish oil-based nutritional supplements. The quantitative analysis of elements with the lowest permitted daily exposure (PDE) values (As, Cd, Hg, Pb) was performed by ICP-MS to demonstrate recovery rates and the efficiency of sample digestion.
Regulatory Background
International Conferenceon Harmonization
The ICH guideline Q3D step 4 has been valid since December 2014 and must be considered for the submission of new drug approvals dating from June 2016 on. For already-approved drug products currently on the market, this guideline has to be followed as of December 2017 (1).
The ICH guideline classifies elemental impurities based on their toxicity and the possibility of their occurrence in drugs products. Elements are classified in four categories: 1, 2A, 2B, and 3. For each element and dosage form, whether oral, parenteral, or inhalation, PDE values are specified.
Particular attention is given to the ubiquitously occurring elements of class 1, the so-called “big four”-cadmium, mercury, lead, and arsenic-as well as class 2A elements such as cobalt, vanadium, and nickel. All of these are highly toxicologically relevant and therefore have the lowest PDEs. For these elements, a risk analysis concerning the possibility of breaching the respective PDE is mandatory, even if these metals have not been added intentionally. Depending on the outcome of this assessment, a justified control strategy has to be defined that can range from the absence of any analysis to periodic studies or routine batch release testing of finished drug products.
United States Pharmacopeia
USP general chapters <232> “Elemental Impurities – Limits” and <233> “Elemental Impurities – Procedures” became official in December 2015 and will replace all references to the old USP general chapter <231> as of January 2018 (2). The limits stated in general chapter <232> are completely aligned with the requirements of ICH Q3D.
For dietary supplements, USP general chapter <2232> has already been made official since August 2013. It refers to USP general chapter <233> concerning the analytical procedure for total element contaminants, which will become applicable in January 2018.
European Pharmacopoeia
The European Pharmacopoeia (Ph. Eur.) Commission decided to reproduce verbatim the ICH Q3D guideline in Ph. Eur. chapter 5.20, which has to be considered for all existing drug products on the European Union (EU) market as of December 2017 (3).
Experimental
To demonstrate the suitability of microwave digestion as a sample preparation technique for elemental impurities analysis, three pharmaceutical products (Table I) were chosen to cover a wide range of different formulations and show varying reaction behaviors during the digestion:
- Aspirin Express tablet (500 mg, Bayer GmbH) with inert silicon dioxide as the excipient,
- Omega-3 capsule (Salus Pharma GmbH) with highly reactive salmon oil, and
- VICKS cold syrup (Procter & Gamble) with three active ingredients and high amounts of alcohol, sugar, and glycerin.
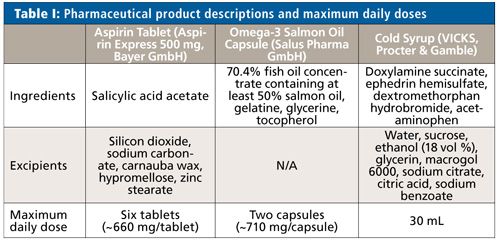
According to USP general chapter <233>, the samples were spiked between 50% and 150% of each element’s target concentration. To evaluate these limits, the respective PDE and the maximum daily dose stated on the package leaflet of the products were taken into consideration. Efficiency of the sample digestion was evaluated by calculating the recovery rates of the measured samples in relation to the theoretical values (unspiked sample + spiked value).
The focus was set on the “big four” elemental impurities-Cd, Pb, As, and Hg-which have the lowest PDE levels. Particularly, As and Hg compounds are analytically demanding because of their potentially volatile behavior.
Instrumentation
All digestions were performed using an Anton Paar Multiwave PRO microwave digestion system equipped with a 24-vessel rotor (Rotor 24HVT50). To demonstrate the comparability of digestion performance, an eight-vessel rotor (Rotor 8NXQ80) was also used for the aspirin and salmon oil samples. Specifications for the two rotor types are summarized in Table II.
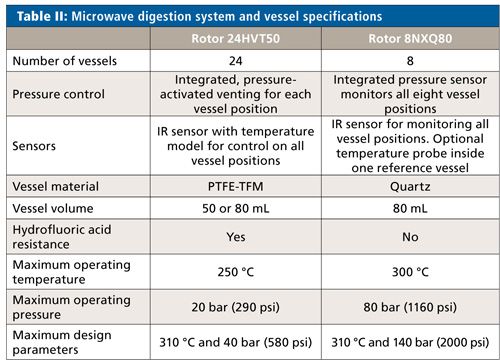
The instrument software complies fully with 21CFR Part 11 requirements, while the optionally available pharmaceutical qualification package (PQP) is a comprehensive instrument qualification document binder that follows a design qualification (DQ)–installation qualification (IQ)–operational qualification (OQ)–performance qualification (PQ) outline according to USP <1058> and GAMP 5.
The quantitative analysis was performed on an Agilent 7900 ICP-MS system, with Ar as the nebulizer gas. Helium was used as the collision gas to compensate for polyatomic interferences when measuring As.
To avoid signal enhancement because of residual carbon, a mixture of 1% CO2 in Ar was added to the nebulizer gas. All solutions were diluted so that they did not exceed 5 µg/L of Hg. A final concentration of 10 µg/L of Ge, In, and Lu was added as internal standards.
Digestion Procedures
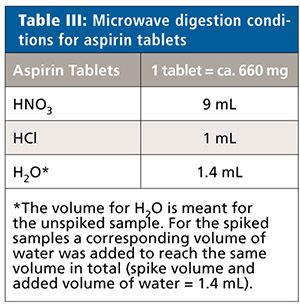
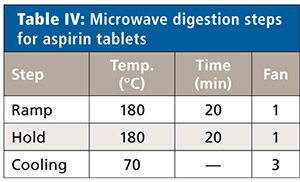
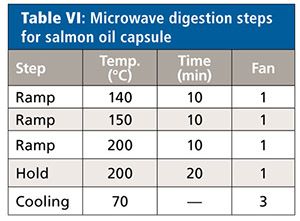
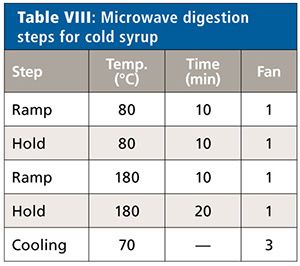
All samples were measured unspiked and spiked at 50%, 100%, and 150% of the target concentration limits of each element (Cd, Pb, As, and Hg). Each sample solution was prepared in triplicate (n = 3). Details of the microwave system settings and digestion conditions for the three samples types are found in Tables III–VIII.
Results
Aspirin Tablets
The absolute quantitation limit was determined as <0.001 µg/mL for each element, considering weighing and dilution of the sample this yields a sample concentration of <0.045 µg/g per sample. Since the unspiked samples did not show any relevant intensity for each of the elements, the theoretical value of the spiked sample was set equal to the spiked value for the calculation of the recovery rates.
Although there was a slight amount of white residue remaining in each of the digestion solutions (probably because of undigested silicon dioxide), the recovery rates for each element lie between 92% and 99% (Table IX). The relative standard deviation for n = 3 does not exceed 5.1%. Since the residues settled completely at the bottom of the sample tubes, aliquots of the clear supernatants were taken for the ICP-MS analyses.
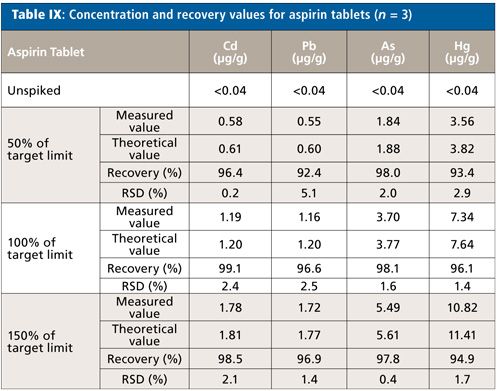
The precipitation disappeared after adding 1 mL of HF to the digestion solution and applying a second digestion program (10 min to 150 °C and 10 min hold). With this additional step no relevant differences were observed-recovery rates between 94% and 105% and relative standard deviations (RSDs) below 2.2% were obtained.
Results of a digestion cycle completed with the eight-vessel rotor also resulted in silica residues, but once more excellent recoveries from 93% to 102% and RSDs lower than 5.6% were achieved.
Because of the satisfactory data achieved on both rotor configurations, it does not matter if the sample is leached or totally digested.
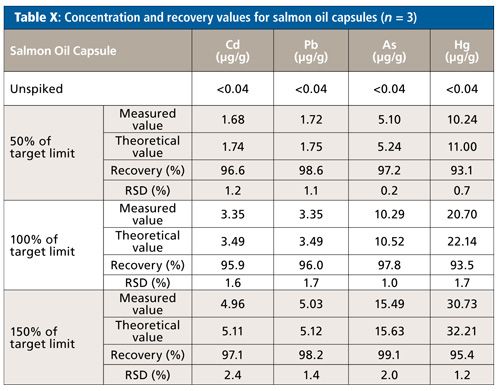
Salmon Oil Capsules
The absolute quantitation limit was determined as <0.001 µg/mL for each element, considering weighing and dilution of the sample this yields a sample concentration of <0.042 µg/g per sample. Since, again, the unspiked samples did not show any relevant intensity for each of the elements, the theoretical value of the spiked sample was set equal to the spiked value for the calculation of the recovery rates.
The recovery rates for each element were between 93% and 99% (Table X). All relative standard deviations lie below 2.4%. The values obtained when repeating the digestion using the eight-vessel rotor configuration did not differ-recovery rates were between 94% and 100% and RSDs were below 1.7%.
Cold Syrup
All digestion solutions showed a slight yellow coloration. For this product the absolute quantitation limit of <0.001 µg/mL leads to <0.0066 µg/g sample for each element based on the weighing and dilution of the sample. Also in this case, the unspiked samples did not show any relevant intensity for each of the elements, and the theoretical value of the spiked sample was set equal to the spiked value for the calculation of the recovery rates.
The recovery rates for each element and spike level once more showed excellent results, yielding between 95% and 104% (Table XI). The relative standard deviations for n = 3 did not exceed 5.1%.
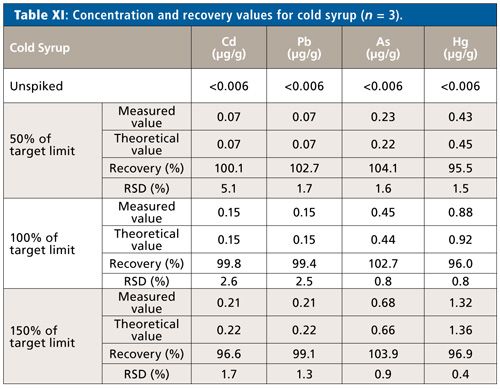
Taking the results of all samples and rotor configurations into consideration, Hg tends to yield the lowest (on average about 95%) and As the highest recovery values (about 101%). Pb recovery rates lie on average at 97% with Cd at 98%. In relation to the absolute figures and limits stated in USP general chapter <233> for method validation (70–150% for spike recovery), these aforementioned recovery variations can be judged as uncritical (Figure 1).
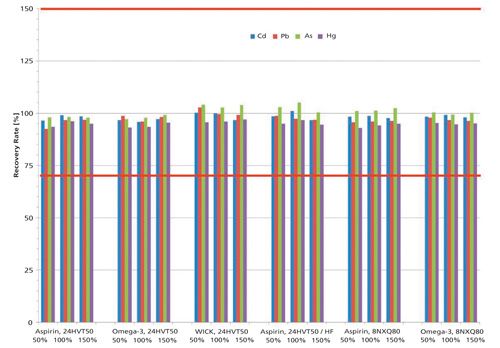
Figure 1: Recovery rates (%) for all samples and rotor configurations.
Regarding RSD, the values are statistically distributed according to sample and element. Each of the four highest RSDs originates from the lowest 50% spiking level (Figure 2) . The limit for the RSD concerning repeatability is stated to be less than 20% in USP general chapter <233>.
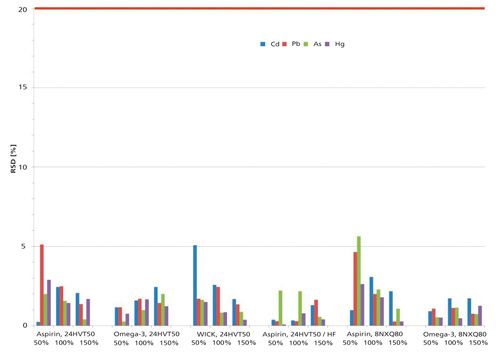
Figure 2: Relative standard deviations (%RSD) for all samples and rotor configurations.
Conclusion
The suitability of microwave-assisted closed-vessel digestion combined with ICP-MS was successfully verified on three different pharmaceutical products. Excellent recovery rates between 92% and 105% combined with very low variations (RSD <5.6%, n = 3) were achieved. The respective limits stated in USP general chapter <233> are 70–150% for spike recovery, and <20% RSD for repeatability.
The microwave-assisted closed-vessel digestion approach represents a reliable, powerful, and fully good manufacturing practice (GMP) compliant sample preparation technique for the determination of elemental impurities according to all current regulatory requirements (ICH, USP, Pharm Eur.).
References
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step_4.pdf.
- US Pharmacopeial Convention; http://www.usp.org/news/usp-establishes-january-1-2018-implementation-date-elemental-impurities-standards.
- European Pharmacopeia Commission;
- .
Reynhardt Klopper is a senior product specialist for analytical and synthetic chemistry at Anton Paar USA, Inc., in Ashland, Virginia. Direct correspondence to: reynhardt.klopper@anton-paar.com

Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.
New AI Strategy for Mycotoxin Detection in Cereal Grains
April 21st 2025Researchers from Jiangsu University and Zhejiang University of Water Resources and Electric Power have developed a transfer learning approach that significantly enhances the accuracy and adaptability of NIR spectroscopy models for detecting mycotoxins in cereals.
Karl Norris: A Pioneer in Optical Measurements and Near-Infrared Spectroscopy, Part II
April 21st 2025In this two-part "Icons of Spectroscopy" column, executive editor Jerome Workman Jr. details how Karl H. Norris has impacted the analysis of food, agricultural products, and pharmaceuticals over six decades. His pioneering work in optical analysis methods including his development and refinement of near-infrared spectroscopy, has transformed analysis technology. In this Part II article of a two-part series, we summarize Norris’ foundational publications in NIR, his patents, achievements, and legacy.