A New Forensic Tool for Chemical Identification: Raman Microscopy
Chemical analysis in the forensic field is different in many aspects from other areas of analysis. The ultimate goal is to identify the sources of evidence, often by matching chemical composition. In this regard, identifying minor elements or trace impurities is as important as identifying main ingredients. In some cases, identifying minor and trace components can be critical to determining that material collected at the site of a crime is identical to material collected in a suspect's environment. In other cases, full identification of trace evidence can be important. Raman microscopy is capable of providing both types of information on minute amounts of material.
Samples are true unknowns that cannot be reproduced and often require multiple analytical methods, but whose amounts are limited. Sample handling must satisfy not only scientific but also legal requirements. Therefore, a preferable analytical technology in the forensic field is one that requires a small amount of sample, can detect trace amounts, makes no contact with the sample during the measurement, does not destroy the sample, and can be combined with other technologies. Raman microscopy is noncontact and nondestructive, and it can characterize sample volumes as small as 0.125 μm3 (1.25 × 10–13 mL). As the name of the technology suggests, Raman microscopy combines Raman spectroscopy (one of the vibrational spectroscopy technologies) and optical microscopy — one can view the sample under a microscope, isolate particulates or locations in the sample, and record a Raman spectrum for chemical identification. Virtually no sample preparation is required, and samples can be measured through containers.
Information provided by Raman microscopy includes chemical identification as well as structure. In the case of small molecular crystals like drugs, Raman also can differentiate crystalline from amorphous materials, and it can differentiate polymorphs and hydrates and solvates. In polymers, Raman can characterize what is called "morphology," which includes orientation of the molecular chains and crystallinity of the polymer. These differences in polymers can provide information about the history of the material and give insights into the penetration and diffusion of small molecules.
The basis of the information in the vibrational spectrum is derived from the binding of atoms in molecules. The larger the number of atoms, the larger is the number of degrees of freedom, and the larger is the number of bands in a spectrum. The total spectral pattern has been termed a "fingerprint," which is the origin of the ability to differentiate different chemical species as well as more closely related materials such as polymorphs of active pharmaceutical ingredients (APIs) (1).
Polymer Identification
A case of potential interest to the forensic community is the ability to differentiate between different fibers. Figure 1 shows the Raman spectra collected from a variety of fibers — both natural and synthetic. These spectra show how the spectra can "fingerprint" the molecular species, and that it is not only possible to differentiate vastly different polymers, but also similar ones such as nylon 6 and nylon 66.

Figure 1
Physical Properties of Polymers
It is also possible to derive more detailed information about the polymer fibers — information related to their history. For instance, polyethylenes exhibit a range of morphology (orientation and crystallinity) based upon how much branched monomer was added to the polymerization mix; this determines whether the polyethylene is high or low density (a physical property). Figure 2 shows spectra of higher and lower density polyethylene. In low-density polyethylene, the amount of residual amorphous content will be higher than in high-density polyethylene. Broad spectral features at 1080 and 1310 cm–1 (marked by arrows) indicate residual amorphous content, which is stronger in the Raman spectrum of lower density polyethylene. The weaker intensity at 1420 cm–1 is another indicator of lower density polyethylene. It is a clear demonstration of the capability of Raman spectroscopy to differentiate very similar materials.
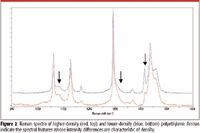
Figure 2
Molecular Orientation in Fibers
In the process of drawing polymers into fiber, it is possible to orient the molecules along the fiber direction (the length of fiber), and also to "crystallize" the polymer (crystallization will depend, among other things, upon the temperature of the material before it solidifies). These properties can be documented in the Raman spectra. Figure 4 shows Raman spectra of the high-density, high molecular weight polyethylene fiber Dyneema (DSM Dyneema, The Netherlands) taken with different polarization conditions (Figure 3). The big differences in intensities in the spectra are due to the high degree of orientation of the polymer in the fiber.
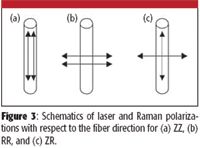
Figure 3
Illicit Drugs
Identification of illicit drugs is straightforward when the Raman spectrum of the suspect material (cocaine) is acquired. Figure 5 shows the Raman spectrum of suspect powder inside a plastic (polyethylene) bag. The spectrum shows spectral features due to both the suspect powder and the bag. A reference spectrum recorded from the bag is then subtracted from this spectrum to isolate the spectral features of the suspect powder. The importance of the ability to acquire the spectrum inside transparent containers is that the evidence is not compromised in any way — there is no possibility of tampering or contamination.
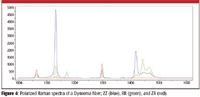
Figure 4
Counterfeit Currency
Figure 6 shows Raman spectra of inorganic additive present in genuine and counterfeit currency. A genuine $20 bill includes anatase, one crystalline form of titanium dioxide, while the counterfeit bill in this case includes calcite, a common form of calcium carbonate. Raman bands marked by arrows are characteristic of anatase (light blue) and calcite (red), which are confirmed by reference spectra recorded from pure anatase and calcite. By identifying the presence of either anatase or calcite, a counterfeit bill is differentiated from a genuine one.

Figure 5
Summary
Using Raman microscopy, identities of a variety of fibers of different chemical composition, density of fibers of the same chemical composition, and orientation and crystallinity of a fiber were differentiated. The identity of an illicit drug (cocaine) was determined by obtaining a Raman spectrum through the container. A counterfeit note was differentiated by identifying inorganic additives. In addition, we have also succeeded in identifying explosives and differentiating similar drugs (cocaine HCl versus crack cocaine, ephedrine versus pseudoephedrine). Because of the ability of the Raman microscope to provide detailed information on a wide range of materials, it is a very promising tool as an addition to the forensic scientist's toolbox.

Figure 6
One of the barriers to implementing Raman microscopy in forensics laboratories has been the capital costs as well as space requirements. However, there are currently new, automated, affordable instruments with the footprint of a standard optical microscope but also including multiple laser excitations.
Fran Adar, George Setola, Sergey Mamedov, and Eunah Lee are with Horiba Jobin Yvon, Edison, New Jersey.
Reference
(1) G. Turrel and J. Corset, eds. Raman Microscopy, Developments and Applications (Academic Press, London, 1996).

Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.
Tomas Hirschfeld: Prolific Research Chemist, Mentor, Inventor, and Futurist
March 19th 2025In this "Icons of Spectroscopy" column, executive editor Jerome Workman Jr. details how Tomas B. Hirschfeld has made many significant contributions to vibrational spectroscopy and has inspired and mentored many leading scientists of the past several decades.