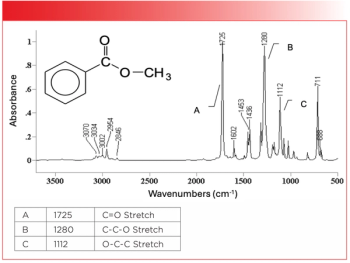
- Spectroscopy-05-01-2019
- Volume 34
- Issue 5
Organic Nitrogen Compounds III: Secondary and Tertiary Amines
A detailed guide to interpreting the infrared spectra of organic nitrogen compounds, including secondary and tertiary amines.
In this third installment of our examination of the infrared spectra of organic nitrogen compounds, we finish our look at the amine family by discussing secondary and tertiary amines. As we see, secondary amines can be distinguished from primary amines based on their infrared spectra. IR can also be used to differentiate between saturated secondary and aromatic secondary amines. We discover that tertiary amines are hard to see using infrared spectroscopy. We examine why, and what to do about it.
As noted in our last installment (1), the amine functional group consists of carbon, hydrogen, and nitrogen atoms, and contain C-H and C-N bonds. Amines are divided into three categories, called primary, secondary, and tertiary amines, based on the number of alpha carbons attached to the amine nitrogen; their molecular structures are seen in Figure 1. Also note that, as the number of alpha carbons goes up, the number of N-H bonds goes down.
Figure 1: The molecular structure of primary, secondary, and tertiary amines. Note the locations of the amine nitrogen and alpha carbon.
Also in the last column (1), we introduced the amine family, and discussed the infrared spectroscopy of the primary amine group. Here we finish our discussion of amines by looking at the structures and infrared spectra of secondary and tertiary amines, and discuss infrared spectroscopy's ability to detect methyl groups attached to nitrogen.
Secondary Amines
Structurally, what sets secondary amines apart from their amine brethren is that they contain one N-H bond, whereas primary amines contain two N-H bonds, and tertiary amines contain no N-H bonds. Also, recall that amines are described using two adjectives: the adjectives primary, secondary, and tertiary describe the number of C-N bonds, and the adjectives saturated and aromatic describe the nature of the carbon(s) attached to the nitrogen. But since secondary amines contain two alpha carbons, it can happen that a molecule can contain both a saturated and an aromatic carbon, as illustrated by the structure of N-methylaniline seen in Figure 2. Is this molecule a saturated or aromatic amine?
Figure 2: The chemical structure of N-methylaniline, a secondary aromatic amine.
The answer is that aromaticity wins. If any of the alpha carbons in an amine are aromatic, the amine is considered aromatic. Thus, N-methylaniline is a secondary aromatic amine.
Recall from the last column (1) that organic nitrogen atoms form somewhat polar N-H bonds, and that these functional groups can form hydrogen bonds.
The hydrogen bonding of amine groups to each other is seen in Figure 3. Because of electronegativity differences, the nitrogen carries a partial negative charge, and the hydrogen a partial positive charge, and they coordinate with each other as shown. Hydrogen bonding is a type of strong intermolecular interaction, and the spectral signature of this results in broadened spectral peaks (1,2).
Figure 3: An illustration of the hydrogen bonding that takes place in amines.
However, because N-H bonds are less polar than -O-H bonds, noting that nitrogen is less electronegative than oxygen, N-H stretching peaks are not as wide as -O-H stretching peaks. We saw this when I first introduced organic nitrogen compounds, and we then compared the peak widths in the spectra of ethanol and propylamine (3). The peak widths in the former are several times broader than in the latter. As previously noted (1–3), both O-H and N-H stretches fall around 3300 cm-1, but we can distinguish them from each other based on peak width differences. One of the ongoing themes of this column series is to integrate peak position, height, and width information to identify functional groups, and distinguishing O-H and N-H stretches is an important example of using peak width information to accomplish this.
One of the things we would like infrared spectroscopy to do is distinguish between primary, secondary, and tertiary amines. This turns out to be relatively easy. As we saw previously (1), primary amines have two N-H bonds, and therefore two N-H stretching peaks; secondary amines have one N-H bond, and hence one N-H stretching peak; and tertiary amines have no N-H bonds, and hence no N-H peaks. The N-H stretching peak of a secondary amine, N-methylcyclohexylamine, is seen in Figure 4, labeled A at 3284 cm-1 (assume all peak positions going forward are in cm-1). Again, note its intermediate peak width and heigfht compared to an O-H stretch, but that the peak is broader than the C-H stretches below 3000. Like primary amines (1), the secondary amine N-H stretch also discloses whether the amine is saturated or aromatic. For saturated secondary amines the N-H stretching peaks falls from 3320 to 3280 as seen in Figure 4, while for aromatic secondary amines it is found near 3400.
Figure 4: The infrared spectrum of N-methylcyclohexylamine, a secondary amine.
The only other useful group wavenumber for secondary amines is the N-H out-of-plane bend, more simply called the N-H wag. Imagine the N-H bond bending above and below the plane of this page. It is labeled D in Figure 4 at 736. In general, this peak falls from 750 to 700. Note that this peak is considerably broader than its neighbors, again due to hydrogen bonding. Unlike the N-H stretch, the secondary amine N-H wagging peak position is not sensitive to whether the amine is saturated or aromatic.
Primary amines also have a wagging vibration involving the NH2 group whose peak falls from 850 to 750 (1), and is similar in size and width to peak D in Figure 4. Thus, in addition to the number of N-H stretching peaks, the position of the N-H wagging peaks of primary and secondary amines can also be used to distinguish them. For primary amines, the wagging peak falls from 850 to 750, whereas for secondary amines it falls from 750 to 700. Table I summarizes the group wavenumbers for secondary amines.
The peak labeled C in Figure 4 is the C-N-C asymmetric stretching peak, and falls from 1180 to 1130 for saturated secondary amines, and from 1350 to 1250 for aromatic secondary amines. Note that this peak's position, in addition to the position of the N-H stretching peak, can be used to distinguish between secondary saturated and secondary aromatic amines. However, note that this peak is relatively weak, and falls in the busy fingerprint region of the spectrum. The reason that this peak is weaker than C-O stretches, which have intense peaks in this region, is because nitrogen is less electronegative than oxygen, and the dipole moment and hence dµ/dx values for C-N stretches are smaller than for C-O stretches. This means this peak is not always reliable, and is an example of a secondary group wavenumber peak that shouldn't be used for identification, but can be used for confirmation.
Tertiary Amines
Tertiary amines are molecules that contain three C-N bonds, and no N-H bonds. The structure of a tertiary amine, N,N-dimethylaniline, is seen in Figure 5.
Figure 5: The chemical structure of N,N-dimethylaniline, a tertiary aromatic amine.
The amine nitrogen here has two saturated and one aromatic carbon attached to it, but, consistent with what we said above, this compound is a secondary aromatic amine.
I have stated previously that the best group wavenumbers for determining the presence of organic nitrogen atoms in a spectrum are the N-H stretching and bending bands (3). Because tertiary amines do not contain these bonds, these peaks are absent from their spectra. Tertiary amines do have C-N stretching peaks that fall from 1250 to 1020, but I am not going to show you any spectra of tertiary amines. That is because the C-N stretching peaks of tertiary amines are weak to medium in intensity, due to the lack of polarity of the C-N bond, and are found in one of the busiest regions of the spectrum. This makes this one peak not particularly useful as a group wavenumber, and it turns out that tertiary amines are one of those functional groups that are particularly difficult to detect via infrared spectroscopy. I have stated several times throughout my columns over the years that we will focus on functional groups that are readily detected by IR spectroscopy; therefore, no more will be said about tertiary amines.
Then how can one detect tertiary amines in a sample? As always, other molecular spectroscopy techniques such as nuclear magnetic resonance (NMR), mass spectrometry (MS), and Raman spectroscopy can be used. However, by making a derivative of your tertiary amine sample, you can still use FT-IR to detect them. To do this mix 1 mL of liquid tertiary amine, or tertiary amine dissolved in an organic solvent, with 1 ml of 50:50 hydrochloric acid (HCl) in ethanol. If there is a tertiary amine present the amine salt will form and precipitate from solution. Collect the precipitate via filtration, dry, and measure its infrared spectrum. Unlike tertiary amines, tertiary amine salts have a bunch of unique and intense infrared features that makes them easy to identify. We will discuss the spectra of amine salts in the next installment of this column.
Methyl Groups Attached to Nitrogen, Oxygen, and Carbon
You may be curious about what the N-methyl designation in N-methylaniline, and N-methylcyclohexylamine stands for, and what the N,N-dimethyl term signifies in the name of N,N-dimethylaniline. Examples of their structures are seen in Figures 2 and 5. N-methyl means there is one methyl or CH3 group attached to a nitrogen, while N,N-dimethyl means there are two methyl groups attached to a nitrogen. This designation is needed to make it clear that these methyl groups are attached to a nitrogen, rather than to one of the other types of atom present in a molecule.
Methyl groups attached to nitrogen are relatively common, and fortunately for us there are some unique peaks to help identify them. If you look closely at the spectrum in Figure 4 of N-methylcyclohexylamine, there is peak labeled B at 2784. Note that this peak is sharp, of medium intensity, and falls just below where typical saturated C-H stretches fall. This peak is due to the symmetric stretching of the N-methyl group. For saturated amines, this peak typically falls from 2805 to 2780, whereas for aromatic amines it is found from 2820 to 2810. When the N,N-dimethyl group is part of a saturated amine, it exhibits two peaks, from 2825 to 2810 and 2775 to 2765. When the N,N-dimethyl group is part of an aromatic amine, it exhibits one peak in the range 2810 to 2790. This information is summarized in Table II.
In previous columns, we have studied the symmetric stretch of a methyl group attached to an oxygen called a methoxy group (4), and the symmetric stretch of methyl groups attached to a carbon (5). Methoxy groups are similar to N-methyl groups in that they also have a sharp, medium intensity low wavenumber methyl symmetric stretch, which falls at 2830 ± 10. The C-CH3 group symmetric stretch falls at 2872 ± 10. This information is summarized in Table III. Note that the methyl symmetric stretches in Table III are sufficiently well separated, so that this peak position can be used to distinguish C-CH3 from O-CH3 from N-CH3. As we go from C-CH3 to O-CH3 to N-CH3, the symmetric methyl stretch gets lower in wavenumber, apparently due to a lowering of the force constants in the methyl group C-H bonds (6).
Conclusions
Secondary amines contain one N-H bond and hence have one N-H stretching peak, as opposed to the two N-H stretching peaks for primary amines. Thus the number of N-H stretching peaks can help distinguish between primary and secondary amines. Secondary amines also have a N-H wag peak different from primary amines, so this peak position can also be used to distinguish these two amine types. Tertiary amines contain no N-H bonds, and hence have no useful infrared peaks. However, making an amine salt derivative and measuring its spectrum will allow infrared spectroscopy to be used to identify tertiary amines. A number of amines have one or two methyl groups attached to the nitrogen, denoted as the N-methyl or N,N-dimethyl groups. These compounds have a unique low wavenumber symmetric stretch that makes them easy to identify.
References
(1) B.C. Smith, Spectroscopy 34(3), 22–25 (2019).
(2) B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, Florida, 1999).
(3) B.C. Smith, Spectroscopy 34(1), 10–15 (2019).
(4) B.C. Smith, Spectroscopy 32 (5), 22–26 (2017).
(5) B.C. Smith, Spectroscopy 33 (4), 18–23 (2015).
(6) B.C. Smith, Spectroscopy 34 (1), 16–23 (2015).
Brian C. Smith, PhD, is founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer reviewed papers, and has written three books on spectroscopy. As a trainer, he has helped thousands of people around the world improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at:
Articles in this issue
over 6 years ago
Review of New Spectroscopic Instrumentation 2019over 6 years ago
Vol 34 No 5 Spectroscopy May 2019 Regular Issue PDFNewsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.