Raman Dynamic Imaging for the Examination of Chemical Reactions
Special Issues
This article investigates Raman imaging for the study of the reaction process involved in the curing of cyanoacrylate adhesives.
This article investigates Raman imaging for the study of the reaction process involved in the curing of cyanoacrylate adhesives. These adhesives cure within a relatively short time, often just a few minutes, and while the chemical reaction that occurs is well understood, it is of interest to examine how the curing process occurs on a microscale across the surface. The reaction rates for "macro" single-point locations and data from micro-imaging areas are compared. Raman imaging of dynamic processes can then be extended to other surface reactions, ranging from biological membranes to photovoltaic devices and more.
The use of "time-resolved" or "dynamic imaging" methods for various samples has been described previously in various texts (1–7). To our knowledge, however, there has not been a comparison of the reaction rates for a macro observation of a chemical kinetics reaction to the same reaction observed on the microscale. It was of interest to us to review the chemical kinetics observed for the curing of a cyanoacrylate adhesive compound in both the macro- and microscales (Figure 1) to see if the reaction rates would compare for both observation methods.
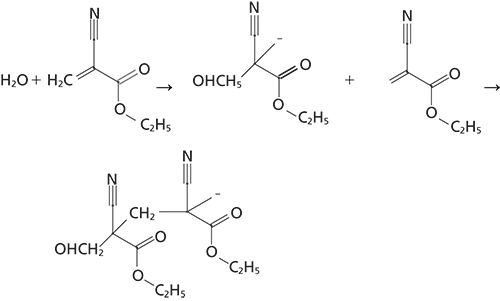
Figure 1: Polymerization reaction sequence of ethylcyanoacrylate adhesive.
Figure 1 outlines the polymerization reaction of the cyanoacrylate adhesive as it cures. The reaction is initialized by an absorption of an OH group from atmospheric water by the adhesive and the polymerization reaction proceeds continuously until the unpolymerized ethylcyanoacrylate molecules are depleted. The analysis of the polymerization reaction was determined by using Raman spectroscopy in a time-resolved mode. The spectra were collected as individual macro- or microspectra of the adhesive in a time-based sequence. By collecting several sets of spectra of the adhesive until the completion of the curing reaction, for as long as 1300–1800 s, the degree of polymerization was observed in the Raman spectra as the reaction progressed.
For the macro experiments, a drop of the cyanoacrylate adhesive was spread on a gold mirror to obtain a uniform layer of the adhesive. Raman sample spectra were collected using a Jasco NRS-5100 instrument with a 5× refractive objective to provide an analysis of a 20-µm area of the sample. Spectra were collected with 532-nm illumination; two accumulations with a 4-s exposure of the charge-coupled device (CCD) were obtained per spectrum. A 1200-gr/mm grating was used, resulting in a spectral resolution of approximately 8.6 cm-1. A time resolution of 10 s between sample scans was used to obtain spectra for approximately 4800 s (80 min).
For the microscopy experiments, the adhesive was prepared in the same manner, but discrete Raman microscope spectra were collected of the adhesive in a "lattice mapping" mode. For the mapping experiments, Raman spectra were collected with the NRS-5100 system and the 50× objective, and discrete spectra with a spatial resolution of approximately 2 µm were obtained. The 532-nm excitation laser was used with the 1200-gr/mm grating, obtaining spectra with a spectral resolution of approximately 8.6 cm-1. Mapping spectra of one accumulation per spectrum with a 6-s exposure of CCD were obtained as the adhesive cured over a total area of 25 × 25 µm using a 3×3 lattice mapping mode. A time resolution of 60 s per sample mapping was used for observations of the mapping area during a total observation time of approximately 4900 s (82 min).
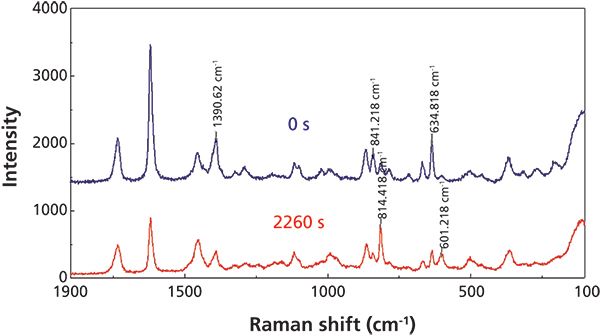
Figure 2: Raman fingerprint spectra of ethylcyanoacrylate adhesive at 0 s (blue) and 2260 s (red).
Results and Discussion
As a result of the overwhelming intensity of the C-H stretch motions, Figure 2 outlines only the fingerprint region of the Raman spectra of the adhesive at the beginning of the reaction (blue trace) and near the end of the curing (red trace), with specific peaks labeled in the traces. The C-C stretch peak at 814 cm-1 and the C-C bend peak at 601 cm-1, are increasing during the reaction, indicating the increase in the number of the CH2-C-CH2 bonds as the polymerization proceeds. Conversely, the C-H bend at 1391 cm-1 and the C=C stretch and bend modes at 841 and 635 cm-1, respectively, are decreasing as bonds associated with the C=C group are rearranged during the curing reaction.
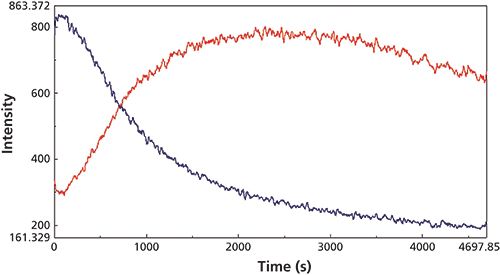
Figure 3: Extracted Raman scattering intensity traces observed during the macro reaction for the C-H bend at 1391 cm-1 (blue) and the C-C stretch at 814 cm-1 (red) Raman peaks.
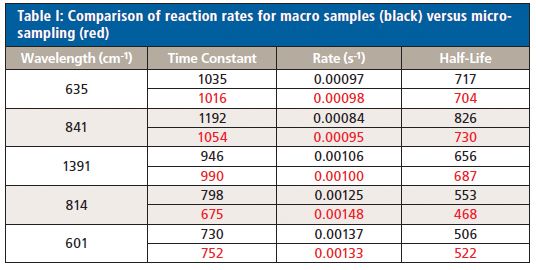
The changes in the vibrational modes were observed for multiple examples of the cyanoacrylate curing reaction and the reaction kinetics calculated for the increasing and decreasing peak intensity values in the Raman spectra for the 1391 cm-1 (C-H bend) and 814 cm-1 (C-C stretch) peaks (Figure 3) and the 841 (C-C stretch), 635 (C=C bend), and 601 cm-1 (C-C bend) peaks for the macro experiment. Additional plots of the 1391 cm-1 (C-H bend) and 806 cm-1 (C-C stretch) peaks (Figure 4) and the 841 cm-1 (C-C stretch) and 601 cm-1 (C-C bend) (Figure 5) peaks for the micro experiment are also displayed. These figures all show the increasing and decreasing intensity values for the vibrational motions outlined earlier. The reaction kinetics values are calculated for these peak intensity changes using the Reaction Rate Calculation software available in the Spectra Manager software, and the results for these calculations are displayed as Table I.
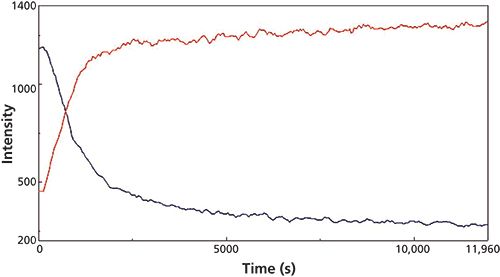
Figure 4: Extracted Raman peak intensity traces observed during the micro reaction for the 1391 cm-1 (C-H bend, red) and 814 cm-1 (C-C stretch, blue) Raman peaks.
As outlined in Table I, the macro- and microsampling methods provide reaction rate values for the time constant, reaction rate, and reaction half-life values calculated using the Reaction Rate Calculation software that agree well within each experiment.
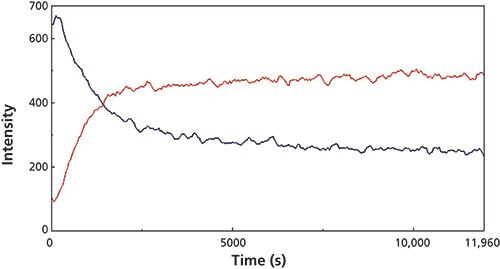
Figure 5: Extracted Raman peak intensity traces observed during the micro reaction for the C-C stretch peak at 841 cm-1 (blue) and the C-C bend mode at 601 cm-1 (red) Raman peaks.
Conclusions
The macro- and microsampling methods for observation of an ethylcyanoacrylate adhesive curing reaction can provide useful kinetics data for the adhesive curing. The reaction data calculated from the Raman kinetics spectra compares quite well with the micro observation method. These experiments demonstrate that additional information can be gained by using a Raman imaging method so that the reaction kinetics on a microscale can be investigated for other chemical systems. In this instance, a standard commercial Raman instrument was utilized for the imaging experiments and no special instrumentation or accessories were required.
Richard A. Larsen is a spectroscopy applications specialist with Jasco, Inc., in Easton, Maryland. Direct correspondence to: rlarsen@jascoinc.com
References
(1) C.A. Coutts-Lendon, N.A. Wright, E.V. Miseo, and J.L. Koenig, J. Con. Rel. 93, 223 (2003).
(2) G. Srinivasan and R. Bhargava, Spectrosc. 22, 30 (2007).
(3) T. Tadokoro, T. Fukazawa, and H. Toriumi, Jpn. J. Appl. Phys. 36, 1207 (1997).
(4) R. Bhargave and I.W. Levin, Appl. Spectrosc. 57, 357 (2003).
(5) R. Bhargava and I.W. Levin, Macromolecules 36, 92 (2003).
(6) R. Bhargava and I.W. Levin, Appl. Spectrosc. 58, 995 (2004).
(7) H. Sugiyama, J. Koshoubu, S. Kashiwabara, T. Nagoshi, R.A. Larsen, and K. Akao, Appl. Spectrosc. 62, 17 (2008).

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.