Resolving Analytical Challenges in Pharmaceutical Process Monitoring Using Multivariate Analysis Methods: Applications in Process Understanding, Control, and Improvement
Multivariate analysis (MVA) refers to an assortment of statistical tools developed to handle situations in which more than one variable is involved. MVA is indispensable for data interpretation and for extraction of meaningful data, especially from fast acquisition instruments and spectral imaging techniques. This article reviews trends in the application of MVA to pharmaceutical manufacturing and control. The MVA models most commonly used in drug analysis are compared. The potential of MVA to resolve analytical challenges, such as overcoming matrix effects, extracting reliable data from dynamic matrices, clustering data into meaningful groups, removing noise from analytical response, resolving spectral overlaps, and providing simultaneous analysis of multiple components, are tackled with examples. Industrial applications of MVA capabilities are described, with special emphasis on process analytical technology (PAT) and how MVA can aid in process understanding and control. A scheme for selecting an MVA model according to the available data and the required information is proposed.
Modern pharmaceutical manufacturing involves the integration of analytical chemistry into critical steps of the manufacturing process for real-time monitoring and control. This integration is essential, especially in continuous manufacturing schemes, to ensure uniform product quality and process robustness, and to reduce manufacturing cost. The continuous monitoring required in a continuous manufacturing environment is challenging for conventional analytical methods. To resolve such challenges, instruments that allow fast and simultaneous capture of multiple data points are preferred, such as near infrared (NIR), Fourier transform infrared (FT-IR), and Raman spectroscopy; mass spectrometry (MS) and UV-visible (UV-vis) photodiode array detectors that capture multiple wavelengths are also commonly recruited. To interpret the complicated analytical signal of these tools, multivariate regression analysis (MVA) is necessary.
MVA refers to an assortment of statistical tools developed to handle situations in which more than one variable is involved (1–5). MVA can be applied to resolve numerous analytical challenges in the pharmaceutical industry, such as eliminating sample extraction steps during process monitoring, determining the drug substance in an unknown solution that is constantly changing or in a dynamic matrix, minimizing the impact of instrumental fluctuations, and enabling the simultaneous determination of more than one component in a certain matrix.
Selecting the appropriate MVA model has always been challenging task (2,3,6). In this review, the MVA models most commonly used in drug analysis are discussed and compared. A scheme for selecting a suitable MVA model is proposed (Figure 1). The available input data and the type of information required determine whether a supervised or unsupervised model, and which algorithm, should be used. Supervised models are those in which the data include input variables and an output variable, and an algorithm is applied to learn the relationship between the variables and train the model to predict the output when new data are provided. Supervised models can be used for either regression or clustering. Examples of supervised models are multiple linear regressions (MLR), classical least squares (CLS), partial least squares (PLS), artificial neural networks (ANNs), and locally weighted regression (LWR). Unsupervised models are used when the available data are only the “X” input variables. They can be used for clustering and association. Examples of unsupervised models are principal component analysis (PCA) and hierarchical cluster analysis (HCA) (4,5).
FIGURE 1: A proposed scheme for selecting the optimal MVA model for pharmaceutical analysis.
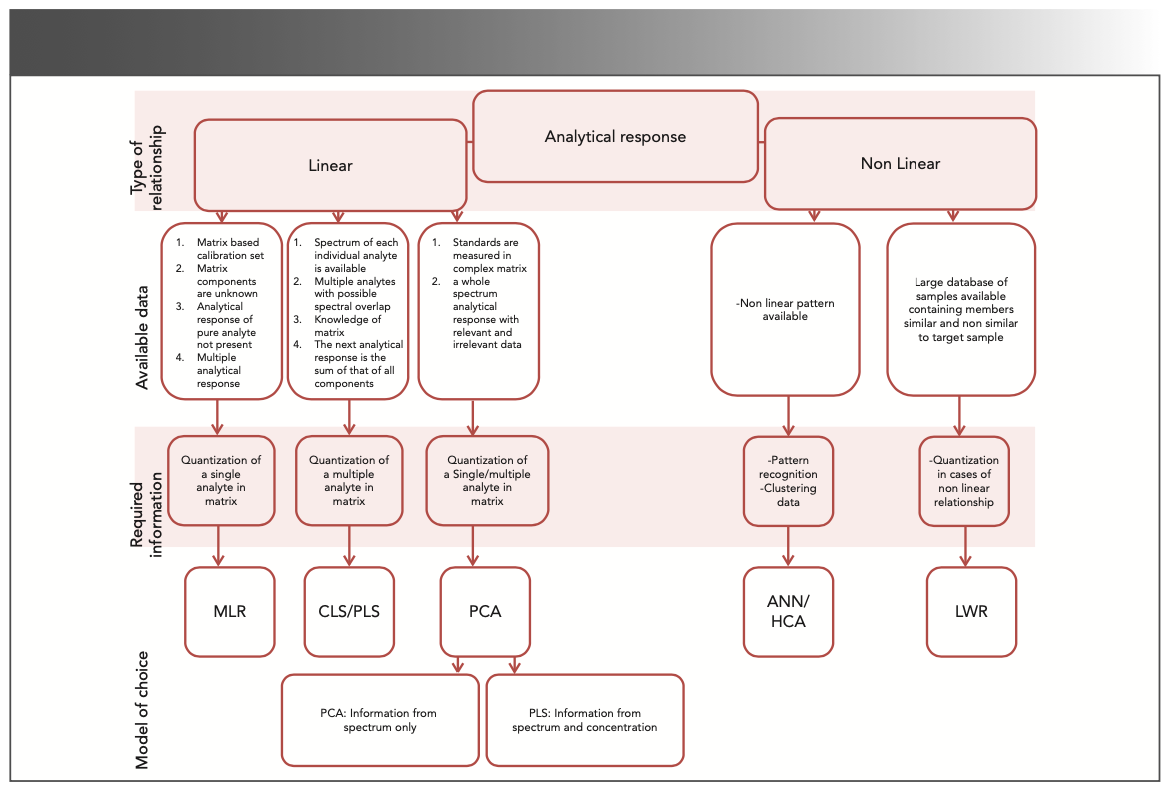
CLS and MLR are used for discrete measurements, such as absorbance at certain wavelengths. CLS can determine multiple analytes even in the presence of interfering matrix components, but all spectrally active ingredients should be fully known and modeled during calibration (7). MLR is best applicable for determining a single analyte in the presence of specific matrix components. For MLR, calibration can be done by varying the single analyze concentration in the sample matrix, which allows test samples to be directly analyzed without extraction (8). In both methods, irrelevant spectral information may affect the model prediction accuracy.
In more complicated models where the analytical signal is a full range that may contain both relevant and irrelevant information, PLS can be applied. PLS finds the latent variables. These latent variables relate to the largest covariance between the signal and response matrices (9–11). In this context, PLS is considered another type of supervised learning. It’s worth mentioning that CLS is considered the first generation of the PLS method (12). ANN (13) and LWR (14) can work well in nonlinear models.
The unsupervised multivariate methods are PCA and HCA. In both techniques, a correlation matrix (or sometimes a covariant matrix) is usually computed between the different input x–variables (factors). In PCA particularly, the number of generated principal components is equivalent to the number of input variables. Importantly, the variation in the studied and investigated data and scale ranges is usually normalized to an important statistical parameter, the unit variance (1/SD), prior to analysis (where SD is standard deviation). The correlation matrix is utilized to determine the data principal components, which are actually the eigenvectors of the matrix. Accordingly, the computed eigenvectors are ranked according to their eigenvalues (scalar values) and consequently, the eigenvector (which is actually a geometrical direction) with the largest eigenvalue is selected as the main principal component, followed by the vector having the second eigenvalue in magnitude, and so on (15).
Similarly, HCA depends on the correlation matrix to generate its specific dendrogram (dendron means tree in Latin). Both techniques help in clustering the data into meaningful groups for pattern recognition applications (12).
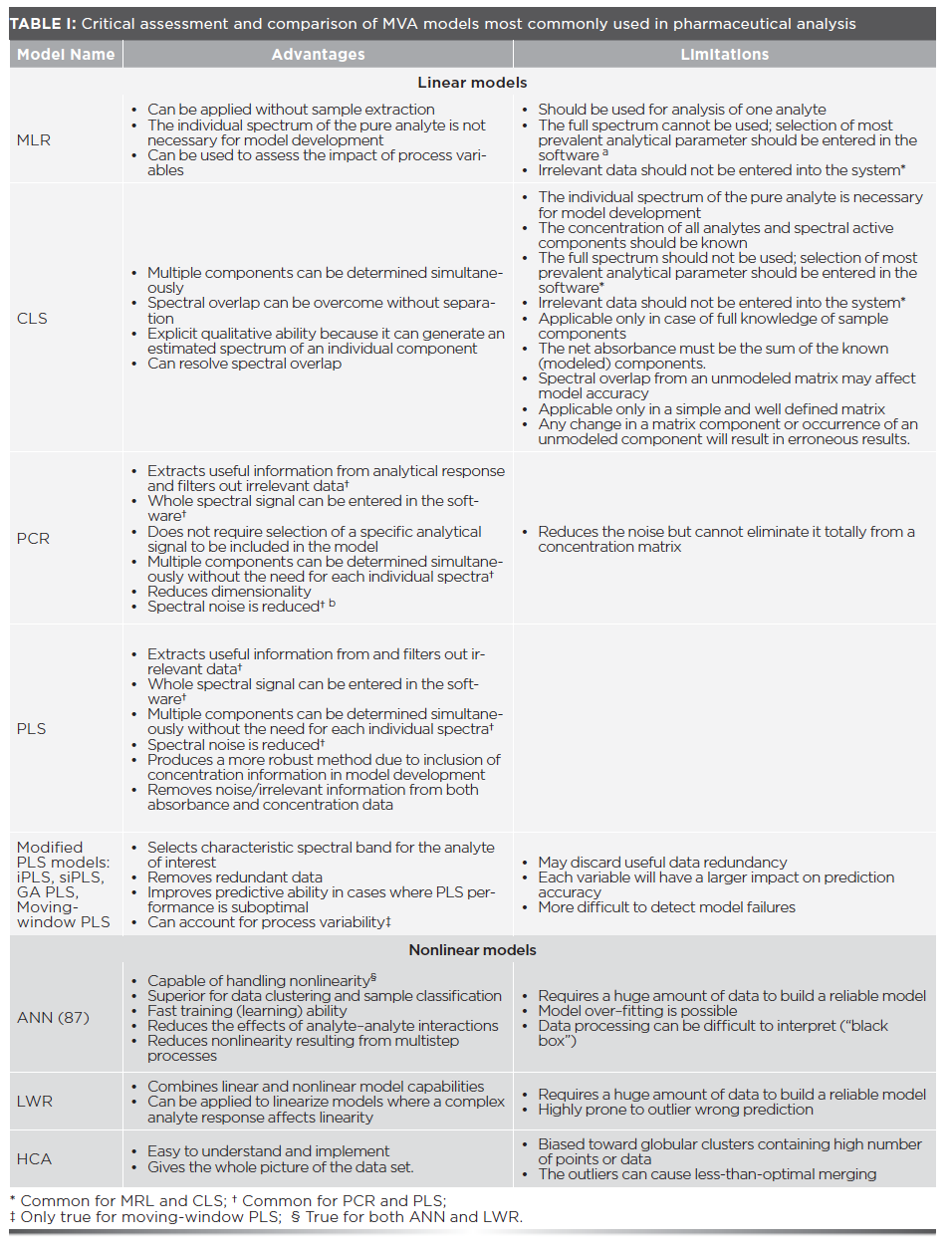
When information is scarce—a situation usually encountered in the early stages of screening—unsupervised methods, such as HCA and PCA, are preferred. In the later stages of screening, supervised methods such as MLR, PLS, and ANN are often used to predict specific sample properties while still using the former methods to detect similarities and dissimilarities in the data (16). A comparison of MVA techniques discussed in this paper, in terms of advantages and limitations, is presented in Table I. Examples of situations where MVA is analytically useful are discussed.
Eliminating Sample Extraction and Preparation
Eliminating sample extraction steps is necessary in many situations, such as for online, inline, or atline analysis. This would imply recruiting MVA for extracting relevant information from the captured data where the analytical signal contains information from the analyte of interest as well as other interfering materials.
Tablet monitoring using hyperspectral imaging (HSI) is an example of such an application (17). HSI is a nondestructive and fast analysis option that gives information about tablet component homogeneity and distribution as well as the presence of impurities (18–20). It works by simultaneous acquisition of spatial and spectroscopic data from a sample and gathering them into an HSI data cube where the x- and y-axes contain the spatial information while the z-axis contains the spectral information. Collected data are then processed to correct baseline drifts, spectral overlap, and multiplicative scattering; then, the region of interest (ROI) that best represents the analyte of interest is chosen with the aid of MVA models such as PCA. Following the identification of the ROI, a quantitative model can be generated either in univariate or multivariate mode.
Another application of MVA in chemical imaging is in the detection of counterfeit pharmaceuticals. The following techniques were applied for the detection of counterfeit Viagra tablets: Raman microscopy imaging for data acquisition, PCA for determination of the ROI, and K–nearest neighbor (KNN) for data classification. KNN is considered a basic classification technique. It is a supervised learning model that has great application in classification and pattern recognition. KNN functions by determining the Euclidian distances (the square root of the sum of the square differences) between the test sample and the standards available in the training set. It determines the K number of points that is closest to the test data. The test is identified based on the group to which the majority of the K objects belong (21).
Another example of the use of MVA is seen in HSI of the UV region from 225 to 400 nm for the determination of ibuprofen, acetylsalicylic acid, and paracetamol in tablets. The collected data were deconvoluted by PCA (22).
Monitoring the content and spatial distribution of salicylic acid in film tablets using FT-Raman mapping coupled to multivariate curve resolution (MCR) has also been described. The MCR assumes that the spectral response is a linear combination of the spectra of the pure components in the system. The model decomposes the instrumental response to the individual spectra and gives distribution maps of the components in the spectral image. In this study, pure spectra of salicylic acid and the other excipients were individually collected. FT-Raman mapping spectra were collected as 16 sample points from the flattened tablet surfaces over an area of 3000 × 3000 μm with a step size of 1000 × 1000 μm2. The ROI was selected based on the characteristic peak for salicylic acid and a salicylic acid distribution map was generated in which the active pharmaceutical ingredient (API) was located and the API concentration is represented by color intensity. Quantitation of the API content was performed by averaging the Raman intensity at 1635 cm−1 (the characteristic peak for salicylic acid) as collected from the 16 data points (23).
The solid state transitions of the API and excipients can be assessed by HSI. An example is the assessment of piroxicam and lactose dehydration on the surface of a tablet using NIR-based HSI using MCR–alternating least squares (ALS) and parallel factor analysis (PARAFAC) (24).
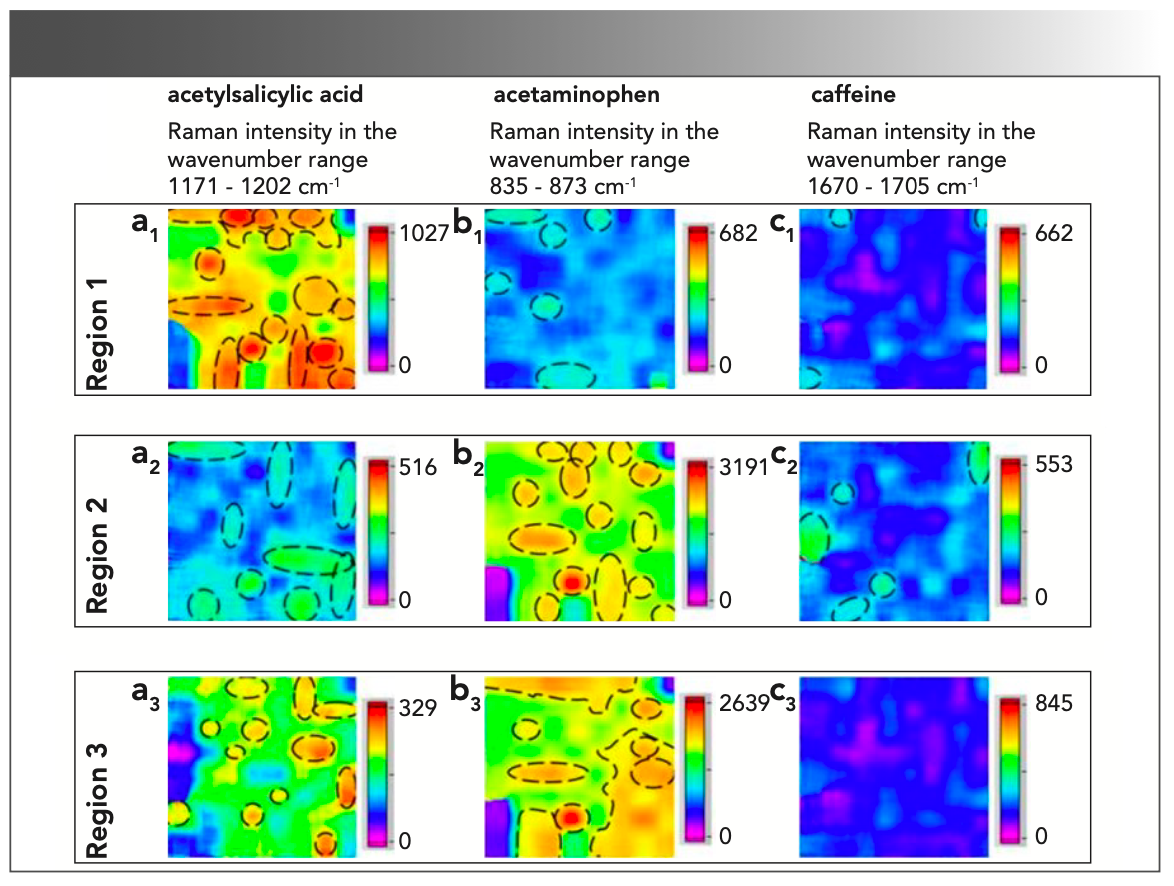
Another example is the application of fiber-array–based Raman HSI for the simultaneous, chemically selective monitoring of particle size and shape of pharmaceutical ingredients—acetylsalicylic acid, acetaminophen and caffeine—in analgesic tablets (Figure 2) (25).
FIGURE 2: Representative Raman HSI of the distribution and particle shapes of (a1, a2, a3) acetylsalicylic acid, (b1, b2, b3) acetaminophen, and (c1, c2, c3) caffeine, in three different regions (1–3) of a tablet (25).
Monitoring blending and analyzing blend components is another analytical challenge that must be performed without extraction in continuous manufacturing. An example is the atline analysis of API in powder blends using FT–NIR where the analytical signal was captured in the range of 4000 to 12,500 cm−1. PLS was used to determine the latent variables that represent the covariance between spectral signal and the amount of API in sample (26). Another example is the determination of hydrochlorothiazide in powder blends by the use of NIR adjunct to PLS. The NIR spectra were recorded for powdered samples in the range of 100 to 2500 nm from which the region 640 to 1780 nm was chosen for the determination. A calibration curve was computed on laboratory-prepared powder blend samples in which additives were included. The procedures were accurately applied to test samples with no sample extraction or preparation (27).
Reducing the consumption of organic solvents is an important benefit of eliminating sample extraction. An example of this concept is the use of thermogravimetric analysis (TGA) for the direct quantitation of ascorbic acid in an effervescent dosage form. In the reported method, a calibration set was prepared using a series of different ascorbic acid concentrations in the presence of additives. The analytical response used was TGA measured at two temperatures for MLR modeling. A relationship was constructed between the ascorbic acid concentration and the TGA measurement at the two selected temperatures in the presence of additives. The method was validated and successfully applied to the determination of ascorbic acid in the dosage form. The method enabled direct analysis of ascorbic acid with no sample pretreatment steps, which reduces the volume of solvents and waste compared to other analytical procedures (28).
Determination of Drug Substance in an Unknown, Constantly Changing, or Dynamic Matrix
The components of a sample change frequently and rapidly during drug manufacturing. Continuous monitoring implies fast analysis to cope with the product evolution and minimizing the effect of variability in the sample components.
NIR is very valuable in this respect. It offers a very fast spectral acquisition method that can be applied for consistent analysis of blend uniformity or monitoring of the dissolution profile using MVA (such as PLS) for data interpretation (26,29). Similarly MVA can be applied to the monitoring of extraction processes (30) and completion of chemical reactions (31).
Minimizing the Impact of Instrumental Fluctuation, Inconsistency, Noise, Irrelevant Data, and Response Overlap
Proper application of an MVA model can correct for instrumental fluctuation and noise by extracting only relevant information. It can also model (qualitatively or quantitatively) spectral data containing overlapped signals. This capability enables the use for previously abandoned analytical tools for quantitative applications.
In this context, X-ray diffraction (XRD) is the most popular tool for polymorphic state assessment. It is a noncontact, nondestructive inspection technique; however, its quantitative application is hindered by the high probability of error because of sample heterogeneity or improper crystal orientation on sampling (28,32). Such obstacles can be overcome by using MVA. A very good example was presented by Otsuka and colleagues where PCA was used to extract four PCs from the XRD measurement that best represent the hydrate content in theophyllin powder. The method was successfully applied to the determination of theophylline monohydrate in theophylline anhydride bulk substance (33). Other examples are presented in other studies (33,34). Another example is the use of FT-IR for estimation of crystalline purity of cimetidin using PCA for data refinement (35).
In this context, it is very important to mention prediction-augmented CLS (PACLS), which is a CLS modification that adds possible spectral variation to the CLS calibration model. Such modification allows identification and removal of spectral variation resulting from a spectrally active component or instrument fluctuation and drifts that were not introduced during model development. The model enables method transfer between different instruments (36,37).
Selecting the Derivative of a Therapeutic Molecule with the Highest Loading on a Nanocarrier
The differences in results between two natural molecules for the treatment of Alzheimer’s disease (curcumin and bisdemethoxycurcumin) were investigated. The difference between these two molecules in terms of their behavior in the biopharmaceutical formulation (loading on poly [lactic-coglycolic acid] [PLGA] nanoparticles) and their therapeutic levels (interaction with certain receptors) were attributed to differences in their main constitutional, electronic, and physicochemical descriptors (38,39). Five or more hypothesized molecules were introduced and their corresponding descriptors were calculated (40). Because the number of descriptors was actually six (more than two dimensions), a multivariate statistics method, PCA, was used to analyze the results and cluster the investigated molecules, according to their descriptors, into meaningful groups (41,42). Interestingly, the docking behavior of the clustered hypothesized molecules with curcumin was very close to that of the parent compound on all of the investigated macromolecules and receptors. Thus, PCA helped address the analytical and manufacturing challenge of determining which of the of the chemical derivatives achieved the highest load on the investigated nanocarrier (the PLGA nanoparticles).
Simultaneous Determination of More than One Component in a Matrix
MVA is useful for resolving spectra of interfering analytes—extracting selective information from unselective data. CLS is especially important in resolving spectral overlaps where during the model development the individual spectrum of each component is input into the system to determine its absorptivity. An example is the simultaneous determination of the spectrally overlapping lamivudine and stavudine (43). Similarly, trimethoprim and sulfamethoxasole were simultaneously determined in an animal bolus preparation without an initial separation step despite their overlapping spectra (44). Another important MVA model for multicomponent analysis is PLS. An example is the simultaneous determination of spectrally overlapping analytes using NIR coupled to PLS without sample pretreatment (45,46).
Handling Nonlinearity in an Analytical Method
LWR and ANN are commonly used to resolve nonlinearity in measurement. In LWR models, a large database of samples is used containing variable amounts of the analyte of interest. On the analysis of a new sample, a higher weight is given to database samples close to the analyzed one to create a modified calibration curve. Adjunct to LWR, PLS can be used to extract the most relevant region in the spectra that best represent the analyte of interest; this reduces spectral noise and irrelevant data in the spectra. An example is the analysis of ibuprofen and magnesium stearate during monitoring of cleaning procedures (47). Another example is the use of LWR– PLS to handle nonlinearity in NIR measurements during determination of API content in granules for tableting (48).
Another application is the simultaneous determination of rifampicin and isoniazide by continuous–flow chemiluminescence (CL) using N–bromo succinic acid (NBS) as a reagent and ANN for data deconvolution (49). Rifampicin degrades in alkaline medium, affecting its reaction with NBS and the resulting CL intensity, but this is not the case for isoniazide. This variation in the reaction kinetics of the two drugs that can be harnessed for their simultaneous analysis, provided proper data management. Meanwhile, both compounds react with each other, resulting in CL nonlinearity. For their simultaneous analysis, alkaline solutions of both drugs were pumped into the CL system and merged with NBS for the reaction to take place. The CL emission was measured over 300 s with a 1 s interval. PCA was used to select the PC that represented the concentration of both components and the PC information was used as an input into the ANN model to improve its performance. For the ANN model, optimum performance was obtained when the input nodes were 20, the iterations were 6000, which represents 20 samples each measured at 300 s (20 x 300), the hidden nodes were 22, and the output was 2 to represent the two active ingredients (49).
Extracting Useful Information from Complex and Overlapping Spectra of Commonly Used PAT Tools
Process analytical technology (PAT) is a system for designing, analyzing, and controlling manufacturing through timely measurements of critical quality attributes (CQA) and critical performance attributes (CPA) of raw and in-process materials and processes with the goal of ensuring final product quality (50). In other words, it is the integration of analytical chemistry into critical steps of a pharmaceutical process for real-time monitoring and control. This integration is essential, especially in continuous manufacturing schemes, to ensure uniform product quality as well as process robustness, and to reduce manufacturing cost.
To achieve real-time monitoring and control, PAT tools are recruited. These are process analyzers (sensors) enabling real-time or nearly real-time analysis. A proper PAT tool should be nondestructive, noninvasive, not require sample withdrawal or contact, and provide fast data acquisition. Ideally handheld instruments and those connected to a probe (inline accessory) are preferable (50).
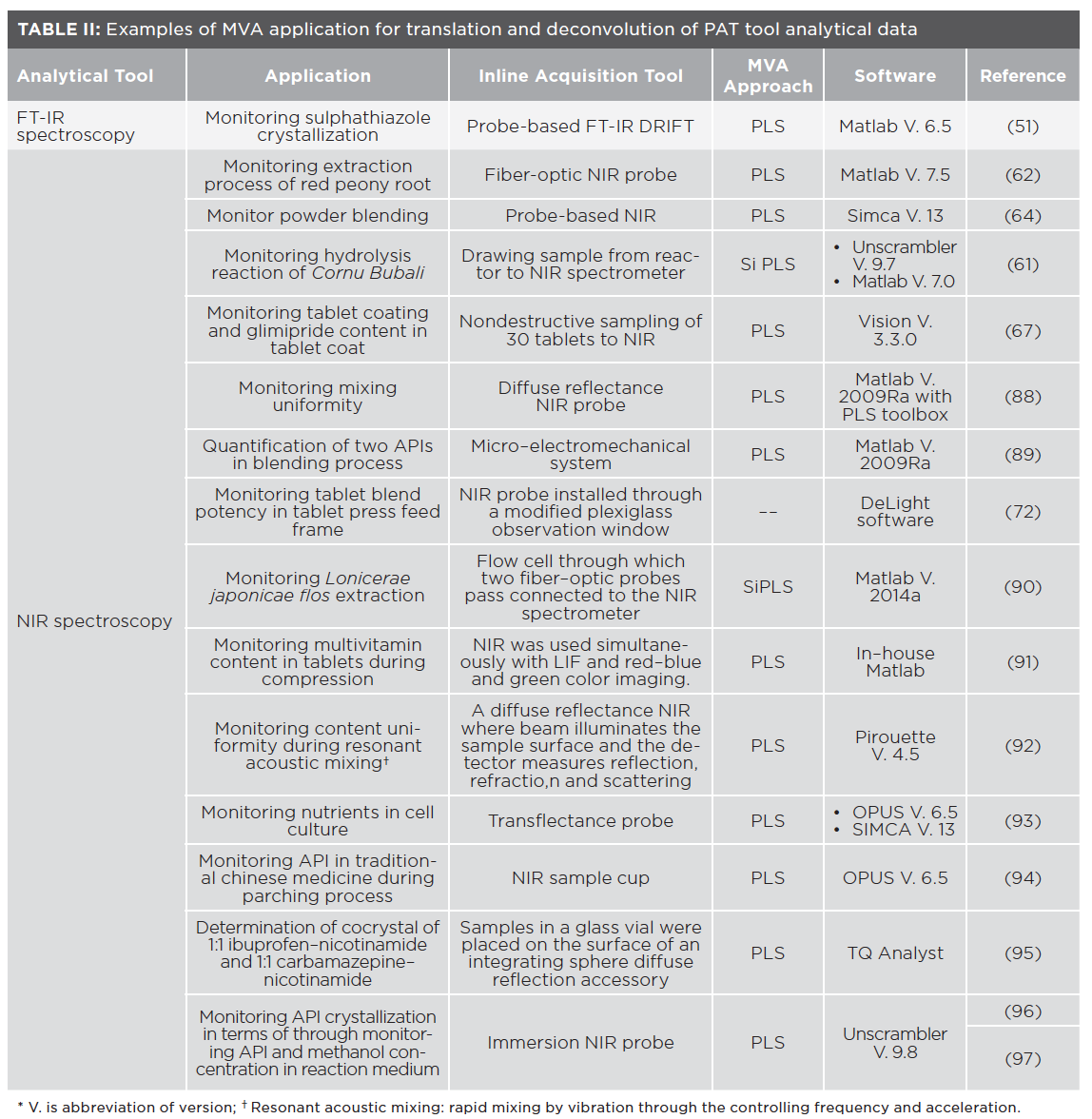
MVA is used with PAT tools to extract useful information from overlapping and complex spectra, analyze multiple components, generate robust data in a continuously changing and dynamic matrix, and handle signal nonlinearity and correlating CPPs affecting the process. The combination of MVA with PAT tools enables adequate process understanding and control. Examples of PAT tools are FT-IR, NIR, and Raman spectroscopy and other whole-spectrum acquisition instruments. Table II includes examples of such applications.
The use of an immersion probe attenuated total reflectance FT-IR (ATR-FT-IR) instrument was presented to monitor the production of sulphathiazole and assess its polymorphic state. The methods were capable of quantifying sulfathiazole product in the range of 0–30 g and predicting the predominant sulphathiazole polymorphs. In both applications, PLS was used to process data (51). Similarly, probe-based ATR-FT-IR spectroscopy was used to monitor a Curtius rearrangement reaction (52). Other examples of recruiting FT-IR for inline monitoring are the flow FT-IR cells suggested by Ley and coworkers (53–56).
NIR is a superior PAT tool. It is the most widely used tool for PAT because it allows fast data acquisition through transparent glass, plastic containers, or (most commonly) through a probe, with acceptable accuracy. NIR radiation can penetrate deep into the sample, allowing extraction of chemical and physical information (57,58). The analysis is nondestructive and thus will not affect final product yield and also will not require a break in the production line for sampling (59). Both NIR imaging and spectroscopy are described in literature. However, 2D imaging data are inadequate for monitoring deep and dynamic events such as blend uniformity. In such cases, the use of NIR probes will be superior, provided proper placement for full data acquisition (60).
Immersion-probe based NIR has been described for inline monitoring of amino acid concentration during hydrolysis of the traditional Chinese medicine Cornu Bubali using PLS for data processing (61). Similarly, in an other example, NIR was used to monitor total solid, paeoniflorin, and benzoic acid during red peony extraction using a PLS regression model (62). In another case, analysis of the chromatographic purification of crude heparin was described, employing NIR monitoring augmented with PLS to select the variable (signal) representing the heparin content (63).
In another case, a probe-based NIR system placed at the tablet feed frame was used to monitor API and excipients during tablet preparation. The spectral data were processed by PLS to give information about powder uniformity (64). A similar application was the real-time determination of acetaminophen in a powder blend and tablets during tablet production (65). Likewise, an inline NIR spectroscopic method for continuous blend potency determination in the feed frame of a tablet press was reported using sodium saccharine as a model active ingredient. In this method the NIR probe was implemented in the feed frame of the rotary tablet press. A PLS model was used for data analysis (66).
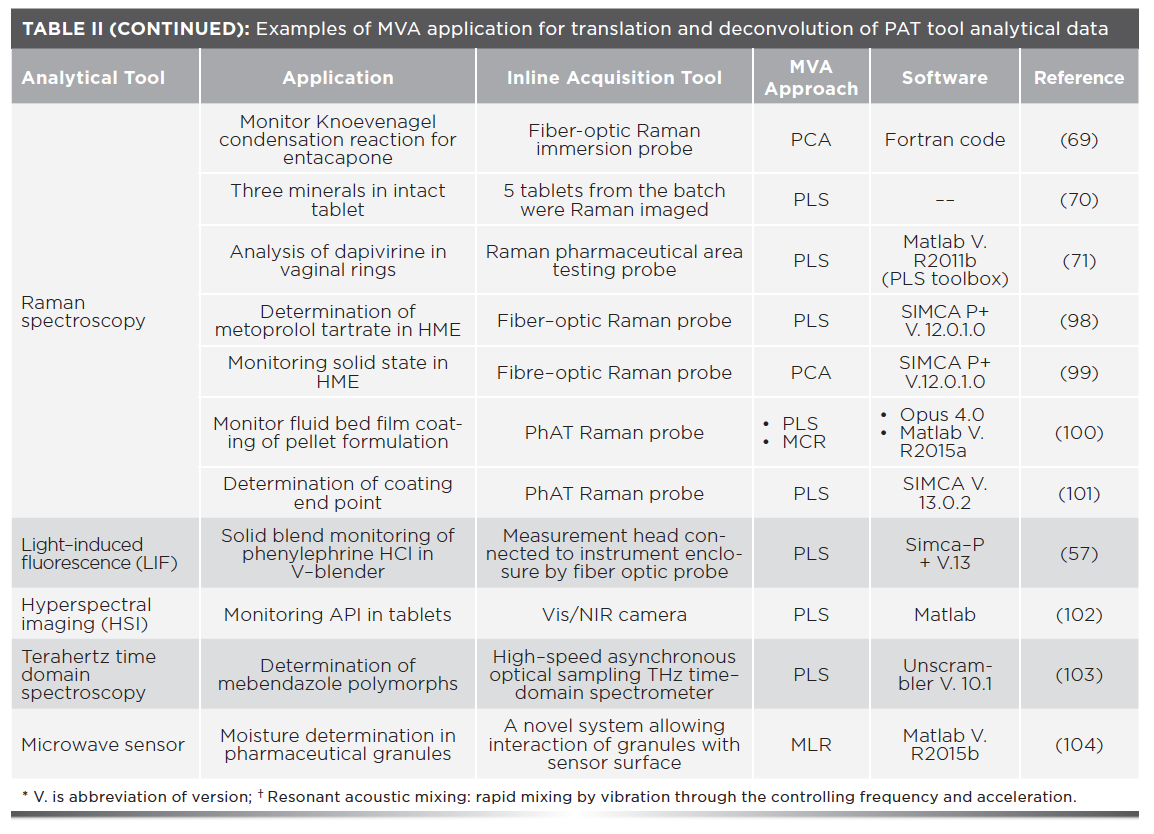
NIR was found to be a successful PAT tool for inline monitoring of a tablet coating using PLS. A fixed-dose tablet of glimepride and metformin was used as an example, where metformin constitutes the tablet core and glimepride exists in the tablet coating. Inline NIR determination of glimepride enabled confirmation of complete coating and identification of the appropriate coating time, which further improved the pharmaceutical process. The process is illustrated in Figure 3 (67).
FIGURE 3: A PAT method using NIR to evaluate a tablet coating process. An NIR probe was mounted in line to monitor glimepride during tablet coating and to identify the coating process endpoint. This information can be used to control the coating process and the amount of drug used. Reprinted with permission from (67). RightsLink license number: 4980260258470.
The use of FT and dispersive NIR instruments to handle continuous process changes such as variation in powder flow rate have been compared (68). In this ex- periment a reflectance NIR probe was connected to FT and dispersive instruments. PLS was used for data processing in both cases. The FT-NIR system showed greater sensitivity and selectivity than the dispersive instrument (68).
Raman spectroscopy is another nondestructive optical tool, with molecular fingerprinting capabilities that enables its application in process monitoring. It has been described for inline Knoevenagel condensation reaction monitoring for entacapone formation using PCA as the associated MVA model. The combination of Raman spectroscopy and PCA enabled faster identification of a reaction end point (69). Determination of minerals in whole tablets has also been described, where Raman spectroscopy was coupled to PLS (70). This technique has also been used for the assessment of dapivirine in vaginal rings (71).
Light–induced fluorescence (LIF) is another emerging PAT tool, and its high sensitivity enables its use for monitoring APIs at very low concentrations. In one example, LIF was mounted using a fiber optic probe into a V–blender. Adjunct to PLS, LIF was able to monitor blend uniformity and accurately determine API content (57).
Handling Process Variability and a Constantly Changing, Dynamic Matrix
Changes in process variables, such as powder rheology, flow rate, and process temperature, may affect beam scattering and thus pose a spectral challenges for PAT tools (72,73). Also, physical properties of measured samples, such as particle size and shape and sample density and compactness may affect the analytical performance of PAT tools (74).
MVA can be used to correct the influence of process variables on accuracy of analytical data (Table III) (74,75). An example is handling the effect of temperature on the accuracy of acquired spectra. This correction can be achieved by augmenting a calibration model to handle temperature-induced variations, as seen in temperature-augmented CLS models (76) and an augmented PLS model (77). An alternative approach is the MVA-assisted selection of a temperature-insensitive variable in the analytical response (78).
Similar to correcting the effect of powder segregation on the accuracy of NIR spectra, inline collection was performed of 500 spectra of a melt extrude, from which 300 were chosen by the PCA model to represent the API content in the melt (79).
Optical path changes may include a change in particle size and sample density, resulting in multiplicative light scattering that in turn affects spectral data reliability. In one example changes in the optical path were corrected by optical pathlength estimation and correction algorithms. The model was applied to correcting NIR spectrum in the presence of variable particles that induced beam scattering (80). Examples also can be found in another study (81).
Beside MVA, proper placement of PAT tools has been suggested as a solution (72). Other researchers have developed statistical models to correct for such signal effects (82). The use of two calibration models has been suggested. The first is a coarse model working over a wide concentration and parameter range throughout a dynamic process (such as blending). The second is a “local” model with fine concentration prediction for a specific stage of blending (58). A recent study applying Raman spectroscopy to inline monitoring of mammalian cell culture production proposed optimizing the Raman acquisition time and including multiple batches in calibrating the model. This was done because acquisition time and batch-related variabilities may affect the integrity of the spectral results (83).
Enhancing Process Understanding and Control
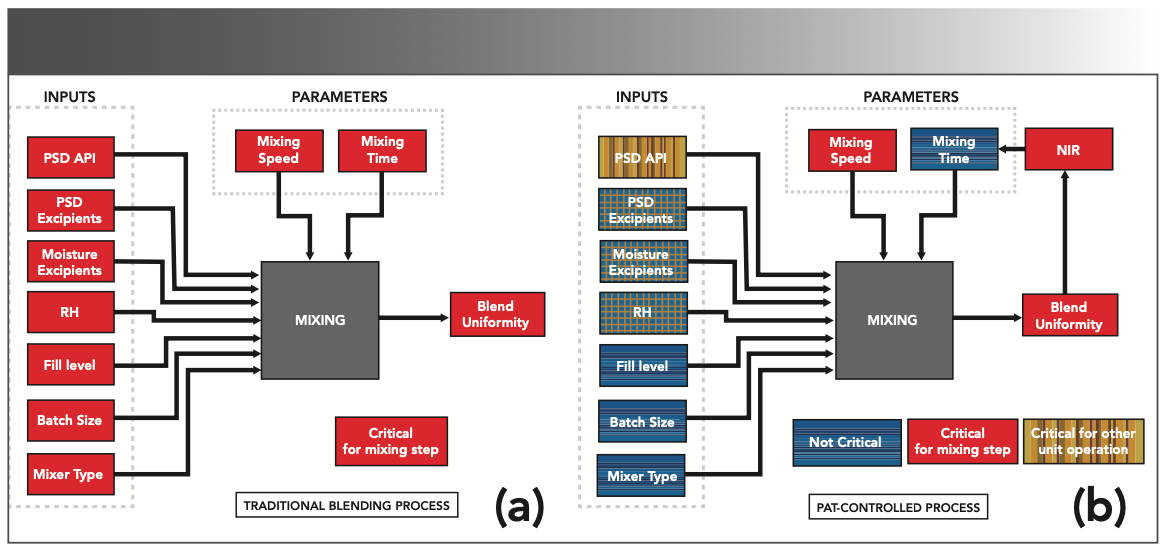
Process understanding helps identify critical points and aspects of a manufacturing process, reducing efforts in process development and scale-up. Figure 4 illustrates a PAT application that reduced the criticality of numerous parameters in a blending process (82). Previously, all the CPPs were considered critical to achieve adequate manufacturing. On applying PAT, an NIR probe was mounted to monitor blend uniformity (the CQA). Mixing speed was identified as the CPP that needed to be adjusted to achieve the desired CQA (82).
FIGURE 4: Illustration of the impact of PAT in evaluating the criticality of blending process parameters. (a) Conventional end-of-line testing where all CPPs were considered critical to achieve adequate manufacturing. In the PAT application (b), NIR was used to monitor blend uniformity, which was identified as the major CQA, and the mixing speed was identified as the CPP that must be controlled. Reprinted with permission from (82); RightsLink license number: 4980260446577.
In another case, PAT enabled understanding the CPPs affecting production of transdermal glimepride liposomal films. Plackett–Burman screening design was applied to quantify the impact of each process variable on the final CQA of the product. MLR was used to correlate CPPs to the CQA (59).
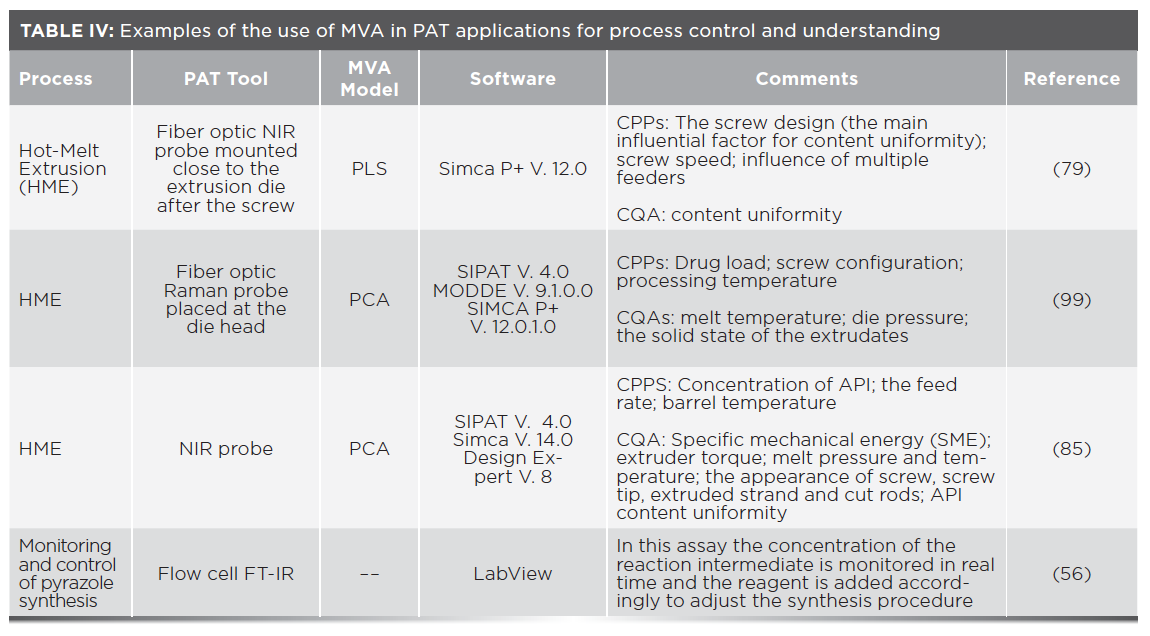
An NIR probe mounted inline was employed to understand CPPs affecting the hot melt extrusion (HME) of vegetable calcium stearate as a matrix carrier and paracetamol as the API using PLS for NIR data interpretation. The results demonstrated that the screw design was the predominant factor affecting uniformity of the preparation, followed by the effect of multiple feeders; the screw speed was shown to not be influential (79).
Process control is another benefit associated with MVA. A clear example of process control is given in the example of a HME process as part of a continuous solid dosage form production process (84). Extrusion is forcing molten material through a die under pressure. The extrudate leaves the die at high temperature and meets chilled rolls, causing its temperature to fall below its glass-transition temperature, leading the extrudate to form brittle glass sheets that are then ground down into smaller flakes for milling followed by blending, addition of excipients, lubrication, and compression into a final product. The HME process combines multiple steps (mixing, melting, degassing, and densification).
In this example FT-NIR spectroscopy was used to monitor form change, assay, and uniformity of the mixed substance (all CQAs) where the CPPs included temperature, mixing, and feed rate. If CQAs are not met, immediate automated discard of out-of-specification material occurs. Then the necessary CPPs are adjusted. This is controlled through a MVA model. If the same process had only monitored by final product inspection, all subsequent stages after the HME would have been completed, requiring that an entire batch of defective final product be discarded (84).
In another example, PAT was applied to evaluate and control a twin-screw HME process for continuous production of tamper–resistant tablets (85). NIR probes were used to monitor content uniformity assisted by PLS for data interpretation. Other CQAs were also monitored, as shown in Table IV. These data were used to control the process by changing CPPs such as barrel temperature or by diverting defective samples from the production line (85). Other important PAT chemometric models such as multivariate image analysis are described in detail in the literature (86).
Conclusions
A plethora of MVA algorithms exist. They encompass linear as well as nonlinear analytical models. Collectively, they represent a solution to many analytical challenges that encountered during pharmaceutical manufacturing. The application of MVA can reduce the cost and environmental hazards of sample preparation and organic solvent usage. A proposed scheme for selecting the optimal MVA model is provided (Figure 1).
MVA offers reduction of sample pretreatment and screening steps and allows the use of nondestructive analytical tools. It enables prediction and determination in nonlinear relationships, clustering into meaningful groups, accurate determination in multi-component samples, removal of instrumental noise, simultaneous determination of many analytes, and processing of overlapping spectra. Additionally, MVA can relate different CQAs with different CPPs and material parameters and allow adequate process understanding and real-time process adjustment and control. These attributes and capabilities make MVA a perfect match for PAT tools for the development and monitoring of pharmaceutical production processes.
References
(1) Bro, R. Multivariate Calibration: What Is in Chemometrics for the Analytical Chemist? Anal. Chim. Acta 2003, 500 (1-2), 185–194. DOI: 10.1016/S0003-2670(03)00681-0
(2) Roggo, Y.; Chalus, P.; Maurer, L.; Lema–Martinez, C.; Edmond, A.; Jent, N. A Review of Near Infrared Spectroscopy and Chemometrics in Pharmaceutical Technologies. J. Pharm. Biomed. Anal. 2007, 44 (3), 683–700. DOI: 10.1016/j.jpba.2007.03.023
(3) Wold, S.; Sjostrom, M.; Eriksson, L. PLSRegression: A Basic Tool of Chemometrics. Chemometrics and Intelligent Laboratory Systems. Chemometr. Intell. Lab Syst. 2001, 58 (2), 109–130. DOI: 10.1016/S0169-7439(01)00155-1
(4) Rajalahti, T.; Kvalheim, O.M. Multivariate Data Analysis in Pharmaceutics: A Tutorial Review. Int. J. Pharm. 2011, 417 (1–2), 280–290. DOI: 10.1016/j.ijpharm.2011.02.019.
(5) B. Swarbrick, Quality by Design in Practice, Multivariate Analysis in the Pharmaceutical Industry; Elsevier, 2018, pp. 125–171.
(6) McNaught, A.D. McNaught, Compendium of Chemical Terminology. Vol. 1669; Blackwell Scientific Publications, 1997.
(7) Franke, J.E. Inverse Least Squares and Classical Least Squares Methods for Quantitative Vibrational Spectroscopy, in Handbook of Vibrational Spectroscopy; John Wiley & Sons, 2006.
(8) Rawlings, J.O.; Pantula, S.G.; Dickey, D.A. Applied Regression Analysis: A Research Tool; Springer Science & Business Media, 2001.
(9) Wang, S.; Xiang, B.; Su, Y.; Tang, Q. Direct Determination of Dichlorvos in Water by Partial Least Square-Discriminant Analysis. Environ. Chem. Lett. 2012, 10, 383–387. DOI: 10.1007/s10311-012-0363-5
(10) Mirghani, M.E.S.; Che Man, Y.B.; Jinap, S.; Baharin, B.S.; Bakar, J. A New Method for Determining Aflatoins in Groundnut and Groundnut Cake Using Fourier Transform infrared Spectroscopy with Attenuated Total Reflectance. J. Am. Oil. Chem. Soc. 2001, 78 (10), 985–992. DOI: 10.1007/s11746-001-0376-y
(11) Abdi, H.; Chin, W.W.; Vinzi, V.E.; Russolillo, G.; Trinchera, L. New Perspectives in Partial Least Squares and Related Methods; Springer Science and Business Media, 2016.
(12) Hathout, R.M.; Metwally, A.A.; Woodman, T.J.; Hardy, J.G. Prediction of Drug Loading in the Gelatin Matrix Using Computational Methods. ACS Omega 2020, 5 (3), 1549–1556. DOI: 10.1021/acsomega.9b03487
(13) J. Schmidhuber, Deep Learning in Neural Networks: An Overview. Neural Networks 2015, 61, 85–117. DOI: 10.1016/j.neunet.2014.09.003
(14) Perez–Guaita, D.; Kuligowski, J.; Quint.s, G.; Garrigues, S.; de la Guardia, M. Modified Locally Weighted—Partial Least Squares Regression Improving Clinical Predictions from Infrared Spectra of Human Serum Samples. Talanta 2013, 107, 368–375. DOI: 10.1016/j.talanta.2013.01.035
(15) Hathout, R.M. Using Principal Component Analysis in Studying the Transdermal Delivery of a Lipophilic Drug from Soft Nano-Colloidal Carriers to Develop a Quantitative Composition Effect Permeability Relationship. Pharm. Dev. Techno. 2014, 19 (5), 598–604. DOI: 10.3109/10837450.2013.813544
(16) Botker, J.; J. Rantanen, J. Multivariate Analysis Supporting Pharmaceutical Research, in Multivariate Analysis in the Pharmaceutical Industry; Elsevier, 2018. pp. 175–184.
(17) Kandpal, L.M.; Cho, B.–K.; Tewari, J.; Gopinathan, N. Raman Spectral Imaging Technique for API Detection in Pharmaceutical Microtablets. Sens. Actuators B Chem. 2018, 260, 213–222 (2018). DOI: 10.1016/j.snb.2017.12.178
(18) Fauteux–Lefebvre, C.; Lavoie, F.B.; Colbert, M.J.; Guay, J.M.; Gosselin, R. Determining the Number of Components for Multivariate Curve Resolution: Case Study Using Raman Mapping of Pharmaceutical Tablets. Microsc. Microanal. 2017, 23 (S1), 1206–1207. DOI: 10.1017/S1431927617006699
(19) Li, C.; Zhang, D.; Slipchenko, M.N.; Cheng, J.–X. Mid-Infrared Photothermal Imaging of Active Pharmaceutical Ingredients at Submicrometer Spatial Resolution. Anal. Chem. 2017, 89 (9), 4863–4867. DOI: 10.1021/acs.analchem.6b04638
(20) L. Coic, P.–Y. Sacre, A. Dispas, A.K. Sakira, M. Fillet, R.D. Marini, P. Hubert and E. Ziemons, Evaluation of the analytical performances of two Raman handheld spectrophotometers for pharmaceutical solid dosage form quantitation. Talanta 2019, 198, 457–463. DOI: 10.1016/j.talanta.2020.120888.
(21) Sacre, P.–Y.; Deconinck, E.; Saerens, L.; De Beer, T.; Courselle, P.; Vancauwenberghe, R.; Chiap, P.; Crommen, J.; De Beer, J.O. Detection of Counterfeit Viagra. by Raman Microspectroscopy Imaging and Multivariate Analysis, J. Pharm. Biomed. Anal. 2011, 56 (2), 454–461. DOI: 10.1016/j.jpba.2011.05.042
(22) Al Ktash, M.; Boldrini, B.; Ostertag, E.; Brecht, M. Characterization of Pharmaceutical Tablets Using UV Hyperspectral Imaging as a Rapid In-Line Analysis Tool. Sensors 2021, 21 (13), 4436. DOI: 10.3390/s21134436
(23) Eksi–Kocak, H.; Ilbasmis Tamer, S.; Yilmaz, S.; Eryilmaz, M.; Boyaci, I.H.; Tamer, U. Quantification and Spatial Distribution of Salicylic Acid in Film Tablets Using FT-Raman Mapping with Multivariate Curve Resolution. Asian J. Pharm. Sci. 2018, 13 (2), 155–162. DOI: 10.1016/j.ajps.2017.07.010
(24) Alexandrino, G.L.; Amigo, J.M.; Khorasani, M.R.; Rantanen, J.; Friderichsen, A.V.; Poppi, R.J. Unveiling Multiple Solid-State Transitions in Pharmaceutical Solid Dosage Forms Using Multi-Series Hyperspectral Imaging and Different Curve Resolution Approaches. Chemom. Intell. Lab. Syst. 2017, 161, 136–146. DOI: 10.1016/j.chemolab.2016.11.004
(25) Frosch, T.; Wyrwich, E.; Yan, D.; Popp, J.; Frosch. T. Fiber-Array-Based Raman Hyperspectral Imaging for Simultaneous, Chemically-Selective Monitoring of Particle Size and Shape of Active Ingredients in Analgesic Tablets. Molecules 2019, 24 (23), 4381. DOI: 10.3390/molecules24234381
(26) Tewari, J.; Strong, R.; Boulas, P. At-line Determination of Pharmaceuticals Small Molecule’s Blending End Point Using Chemometric Modeling Combined with Fourier Transform Near Infrared Spectroscopy. Spectrochim. Acta A Mol. Biomol. 2017, 173, 886–891. DOI: 10.1016/j.saa.2016.10.013
(27) Ferreira M.H.; Braga J.W.; Sena M.M.; Development and Validation of a Chemometric Method for Direct Determination of Hydrochlorothiazide in Pharmaceutical Samples by Diffuse Reflectance Near Infrared Spectroscopy. Microchem. J. 2013, 109, 158–164. DOI: 10.1016/j.microc.2012.03.008
(28) Lang, P.; Kiss, V.; Ambrus, R.; Farkas, G.; Szabo–Revesz, P.; Aigner, Z.; Varkonyi, E. Polymorph Screening of an Active Material. J. Pharm. Biomed. Anal. 2013, 84, 177–183. DOI: 10.1016/j.jpba.2013.06.002
(29) Smetiško, J.; Miljanic, S. Dissolution Assessment of Allopurinol Immediate Release Tablets by Near Infrared Spectroscopy. J. Pharm. Biomed. Anal. 2017, 145, 322–330. DOI: 10.1016/j.jpba.2017.06.055
(30) Wu, Z.; Sui, C.; Xu, B.; Ai, L.; Ma, Q.; Shi, X.; Qiao, Y. Multivariate Detection Limits of On-Line NIR Model for Extraction Process of Chlorogenic Acid from Lonicera Japonica. J. Pharm. Biomed. 2013, 77, 16–20. DOI: 10.1016/j.jpba.2012.12.026
(31) Xintian, Z.; Haibin, Q. Characterisation of the Degradation of Salvianolic Acid B Using an On-Line Spectroscopic Analysis System and Multivariate Curve Resolution. Phytochem Anal. 2012, 23 (2), 103–109. DOI: 10.1002/pca.1330.
(32) Alles., M.; Van Den Berg, F.; Cornett, C.; Jorgensen, F.S.; Halling‐Sorensen, B.; De Diego, H.L.; Hovgaard, L.; Aaltonen, J.; Rantanen, J. Solvent Diversity in Polymorph Screening. J. Pharm. Sci. 2008, 97 (6), 2145–2159. DOI: 10.1002/jps.21153
(33) Otsuka, M.; Kinoshita, H. Quantitative Determination of Hydrate Content of Theophylline Powder by Chemometric X-ray Powder Diffraction Analysis. AAPS PharmSciTech 2010, 11 (1), 204–211. DOI: 10.1208/s12249-009-9357-4
(34) Suda, M.; Takayama, K.; Otsuka, M. An Accurate Quantitative Analysis of Polymorphic Content by Chemometric X-ray Powder Diffraction. Anal. Sci. 2008, 24 (4), 451–457. DOI: 10.2116/analsci.24.451
(35) Calvo, N.L.; Kaufman, T.S.; Maggio, R.M. A Dynamic Thermal ATR-FTIR/Chemometric Approach to the Analysis of Polymorphic Interconversions. Cimetidine as a Model Drug. J. Pharm. Biomed. Anal. 2015, 107, 419–425. DOI: 10.1016/j.jpba.2013.12.036
(36) Haaland D.M.; Melgaard D.K. New Prediction-Augmented Classical Least-Square (PACLS) Methods: Application to Unmodeled Interferents. Appl. Spectrosc. 2000, 54 (9), 1303–1312. DOI: 10.1366/0003702001951228
(37) Haaland D.M.; Melgaard D.K. New Augmented Classical Least Squares Methods for Improved Quantitative Spectral Analyses. Vib. Spectrosc. 2002, 29, 171–175. DOI: 10.1016/S0924-2031(01)00199-0
(38) Magaz, A.; Ashton, M.D.; Hathout, R.M.; Li, X.; Hardy, J.G.; Blaker, J.J. Electroresponsive Silk-Based Biohybrid Composites for Electrochemically Controlled Growth Factor Delivery. Pharmaceutics 2020, 12 (8), 742. DOI: 10.3390/pharmaceutics12080742
(39) Shah, S.A.A.; Firlak, M.; Berrow, S.R.; Halcovitch, N.R.; Baldock, S.J.; Yousafzai, B.M.; Hathout, R.M.; Hardy, J.G. Electrochemically Enhanced Drug Delivery Using Polypyrrole Films. Materials 2018, 11 (7), 1123. DOI: 10.3390/ma11071123
(40) Hathout, R. M.; El-Ahmady, S. H.; Metwally, A. A. Curcumin or Bisdemethoxycurcumin for Nose-to-Brain Treatment of Alzheimer Disease? A Bio/chemo-informatics Case Study. Nat. Prod. Res. 2018, 32 (24), 2873–2881. DOI: 10.1080/14786419.2017.1385017
(41) Ossama, M.; Hathout, R.M.; Attia, D.A.; Mortada, N.D. Enhanced Allicin Cytotoxicity on HEPG-2 Cells Using Glycyrrhetinic Acid Surface-Decorated Gelatin Nanoparticles. ACS Omega 2019, 4 (6), 11293–11300. DOI: 10.1021/acsomega.9b01580
(42) Fagir, W.; Hathout, R.M.; Sammour, O.A.; ElShafeey, A.H. Self-Microemulsifying Systems of Finasteride with Enhanced Oral Bioavailability: Multivariate Statistical Evaluation, Characterization, Spray-Drying and In Vivo Studies in Human Volunteers. Nanomedicine (Lond) 2015, 10 (22), 3373–3389. DOI: 10.2217/nnm.15.123
(43) Mohamed, A.E.–M.I.; Mikre, W. Determination of Lamivudine and Stavudine in Pharmaceutical Preparations Using Chemometrics-Assisted Spectrophotometry. Saudi Pharm. J. 2009, 17 (4), 275–281. DOI: 10.1016/j.jsps.2009.10.003
(44) Ege, H.S.; Ender, Y.; Erdal, D. Spectral Classical Least Square Calibration Approach for the Simultaneous Determination and Stability Test of Sulphadiazine and Trimethoprim in Bolus. J. Anim. Vet. Adv. 2010, 9 (4), 857–861. DOI: 10.3923/javaa.2010.857.861
(45) Melucci, D.; Monti, D.; D’Elia, M.; Luciano, G. Rapid In Situ Repeatable Analysis of Drugs in Powder Form Using Reflectance Near‐Infrared Spectroscopy and Multivariate Calibration. J. Forensic Sci. 2012, 57 (1), 86–92. DOI: 10.1111/j.1556-4029.2011.01945.x
(46) Lin, Y.; Li, W.; Xu, J.; Boulas, P. Development of a NIR-Based Blend Uniformity Method for a Drug Product Containing Multiple Structurally Similar Actives by Using the Quality by Design Principles. Int. J. Pharm. 2015, 488 (1), 120–126. DOI: 10.1016/ijpharm.2015.04.025
(47) Nakagawa, H.; Tajima, T.; Kano, M.; Kim, S.; Hasebe, S.; Suzuki, T.; Nakagami, H. Evaluation of Infrared-Reflection Absorption Spectroscopy Measurement and Locally Weighted Partial Least-Squares for Rapid Analysis of Residual Drug Substances in Cleaning Processes. Anal. Chem. 2012, 84 (8), 3820–3826. DOI: 10.1021/ac202443a
(48) Kim, S.; Kano, M.; Nakagawa, H.; Hasebe, S. Estimation of Active Pharmaceutical Ingredients Content Using Locally Weighted Partial Least Squares and Statistical Wavelength Selection. Int. J. Pharm. 2011, 421 (2), 269–274. DOI: 10.1016/j.ijpharm.2011.10.007.
(49) Li, B.; He, Y.; Lv, J.; Zhang, Z. Simultaneous Determination of Rifampicin and Isoniazid by Continuous-Flow Chemiluminescence with Artificial Neural Network Calibration. Anal. Bioanal. Chem. 2005, 383 (5), 817–824. DOI: 10.1007/s00216-005-0087-5
(50) Kamble, R.; Sharma, S.; Varghese, V.; Mahadik, K. Process Analytical Technology (PAT) in Pharmaceutical Development and its Application. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 212–223.
(51) Pollanen, K.; Hakkinen, A.; Reinikainen, S.–P.; Rantanen, J.; Karjalainen, M.; Louhi–Kultanen, M.; Nystrom, L. IR Spectroscopy Together with Multivariate Data Analysis as a Process Analytical Tool for In-Line Monitoring of Crystallization Process and Solid-State Analysis of Crystalline Product. J. Pharm. Biomed. Anal. 2005, 38 (2), 275–284. DOI: 10.1016/j.jpba.2005.01.009
(52) Xiang, Y.; Lucas, J.; VanAlsten, J.; Li, B.; Preston, B.; Lovdahl, M.; Hayward, C.M. Using Process Analytical Technology (PAT) Tools to Support Flow Chemistry Development and Production. Am. Pharm. Rev. 2012, 15 (3), 56 (2012).
(53) Carter, C.F.; Lange, H.; Ley, S.V.; Baxendale, I.R.; Wittkamp, B.; Goode J.G.; Gaunt, N.L. ReactIR Flow Cell: A New Analytical Tool for Continuous Flow Chemical Processing. Org. Process Res. Dev. 2010, 14 (2), 393–404. DOI: 10.1021/op900305v
(54) Qian, Z.; Baxendale, I.R.; Ley, S.V. A Continuous Flow Process Using a Sequence of Microreactors with In-line IR Analysis for the Preparation of N,N-Diethyl-4-(3-fluorophenylpiperidin-4-ylidenemethyl) benzamide as a Potent and Highly Selective δ-Opioid Receptor Agonist. Chem. Eur. J. 2010, 16 (41), 12342–12348. DOI: 10.1002/chem.201002147
(55) Brodmann, T.; Koos, P.; Metzger, A.; Knochel, P.; and Ley, S.V. Continuous Preparation of Arylmagnesium Reagents in Flow with Inline IR Monitoring. Org. Process Res. Dev. 2011, 16 (5), 1102–1113. DOI: 10.1021/op200275d
(56) Lange, H.; Carter, C.F.; Hopkin, M.D.; Burke, A.; Goode, J.G.; Baxendale, I.R.; Ley, S.V. A Breakthrough Method for the Accurate Addition of Reagents in Multi-Step Segmented Flow Processing. Chem. Sci. 2011, 2 (4), 765–769. DOI: 10.1039/C0SC00603C
(57) Guay, J.M.; Lapointe–Garant, P.–P. ; Gosselin, R.; Simard, J.–S.; Abatzoglou, N. Development of a Multivariate Light-Induced Fluorescence (LIF) PAT Tool for In-Line Quantitative Analysis of Pharmaceutical Granules in a V-Blender. Eur. J. Pharm. Biopharm. 2014, 86 (3), 524–531. DOI: 10.1016/j.ejpb.2013.12.013
(58) Sibik, J.; Chalus, P.; Maurer, L.; Murthy, A.; Krimmer, S. Mechanistic Approach in Powder Blending PAT: Bi-Layer Mixing and Asymptotic End Point Prediction. Powder Technol. 2017, 308, 306–317. DOI: 10.1016/j.powtec.2016.12.038
(59) Ahmed, O.A.A.; Kurakula, M.; Banjar, Z.M.; Afouna, M.I.; Zidan, A.S. Quality by Design Coupled with Near Infrared in Formulation of Transdermal Glimepiride Liposomal Films. J. Pharm. Sci. 2015, 104 (6), 2062–2075. DOI: 10.1002/jps.24448
(60) El-Hagrasy, A.S.; Morris, H.R.; D’Amico, F.; Lodder, R.A.; Drennen, J.K. Near-Infrared Spectroscopy and Imaging for the Monitoring of Powder Blend Homogeneity. J. Pharm. Sci. 2010, 90 (9), 1298–1307. DOI: 10.1002/jps.1082
(61) Wu, Z.; Peng, Y.; Chen, W.; Xu, B.; Ma, Q.; Shi, X.; Qiao, Y. NIR Spectroscopy as a Process Analytical Technology (PAT) Tool for Monitoring and Understanding of a Hydrolysis Process. Bioresour. Technol. 2013, 137, 394–399. DOI: 10.1016/j.biortech.2013.03.008
(62) Wu, Y.; Jin, Y.; Li, Y.; Sun, D.; Liu, X.; Chen, Y. NIR Spectroscopy as a Process Analytical Technology (PAT) Tool for Online and Real-Time Monitoring of an Extraction Process. Vib. Spectrosc. 2012, 58, 109–118. DOI: 10.1016/j.vibspec.2011.10.006
(63) Zang, H.; Wang, J.; Li, L.; Zhang, H.; Jiang, W.; Wang, F. Application of Near-Infrared Spectroscopy Combined with Multivariate Analysis in Monitoring of Crude Heparin Purification Process. Spectrochim. Acta A Mol. Biomol. 2013, 109, 8–13. DOI: 10.1016/j.saa.2013.02.018
(64) Wahl, P.R.; Fruhmann, G.; Sacher, S.; Straka, G.; Sowinski, S.; Khinast, J.G. PAT for Tableting: Inline Monitoring of API and Excipients via NIR Spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87 (2), 271–278. DOI: 10.1016/j.ejpb.2014.03.021
(65) Jarvinen, K.; Hoehe, W.; J.rvinen, M.; Poutiainen, S.; Juuti, M.; Borchert, S. In-line Monitoring of the Drug Content of Powder Mixtures and Tablets by Near-Infrared Spectroscopy During the Continuous Direct Compression Tableting Process. Eur. J. Pharm. Biopharm. 2013, 48 (4), 680–688. DOI: 10.1016/j.ejps.2012.12.032
(66) Fien De Leersnyder, F.; Hasna Djalabi, H.; Valerie Vanhoorne, V.; Bernd Van Snick, B.; Hong, K.; Hammond, S.; Yang Liu, A.; Ziemons, E.; Vervaet, C.; De Beer, T. Development and Validation of an In-Line NIR Spectroscopic Method for Continuous Blend Potency Determination in the Feed Frame of a Tablet Press. J. Pharm. Biomed. Anal. 2018, 151, 274–283. DOI: 10.1016/j.jpba.2018.01.032
(67) Kim, D.W.; Park, J.B.; Lee, S.H.; Weon, K.Y. Development of a Process Analytical Technology (PAT) Method Using Near-Infrared Spectroscopy System for Evaluating an Active Coating Process for a Low-Dose Drug. J. Drug Deliv. Sci. Technol. 2017, 39, 8–15. DOI: 10.1016/j.jddst.2017.02.008
(68) Shi, Z.; McGhehey, K.C.; Leavesley, I.M.; Manley, L.F. On-line Monitoring of Blend Uniformity in Continuous Drug Product Manufacturing Process--The Impact of Powder Flow Rate and the Choice of Spectrometer: Dispersive vs. FT. J. Pharm. Biomed. Anal. 2016, 118, 259–266. DOI: 10.1016/j.jpba.2015.11.005
(69) Novak, P.; Kišić, A.; Hrenar, T.; Jednačak, T.; Miljanić, S.; Verbanec, G. In-Line Reaction Monitoring of Entacapone Synthesis by Raman Spectroscopy and Multivariate Analysis. J. Pharm. Biomed. Anal. 2011, 54 (4), 660–666. DOI: 10.1016/j.jpba.2010.10.012
(70) Salvas, J.; Simard, J.S.; Abatzoglou, N. Raman Spectroscopy to Analyze Intact Pharmaceutical Tablets: Factors Influencing MVPM-based PAT Methods. Am. Pharm. Rev. 2010, 13 (3), 46.
(71) Lyndgaard, L.B.; Sp.ngberg, R.; Gilmour, C.; Lyndgaard, C.B.; Berg, F. A Process Analytical Approach for Quality Control of Dapivirine in HIV Preventive Vaginal Rings by Raman Spectroscopy. J. Raman Spectrosc. 2014, 45 (2), 149–156. DOI: 10.1002/jrs.4433
(72) Ward, H.W.; Blackwood, D.O.; Polizzi, M.; Clarke, H. Monitoring Blend Potency in a Tablet Press Feed Frame Using Near Infrared Spectroscopy. J. Pharm. Biomed. Anal. 2013, 80, 18–23. DOI: 10.1016/j.jpba.2013.02.008
(73) Islam, M.T.; Scoutaris, N.; Maniruzzaman, M.; Moradiya, H.G.; Halsey, S.A.; Bradley, M.S.A.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. Implementation of Transmission NIR as a PAT Tool for Monitoring Drug Transformation During HME processing. Eur. J. Pharm. Biopharm Anal. 2015, 96, 106–116. DOI: 10.1016/j.ejpb.2015.06.021
(74) Chen, Z.; Lovett, D.; Morris, J. Process Analytical Technologies and Real Time Process Control: A Review of Some Spectroscopic Issues and Challenges. J. Process. Control 2011, 21 (10), 1467–1482. DOI: 10.1016/j.jprocont.2011.06.024
(75) Wulfert, F.; Kok, W.T.; Smilde, A.K. Influence of Temperature on Vibrational Spectra and Consequences for the Predictive Ability of Multivariate Models. Anal. Chem. 1998, 70 (9), 1761–1767. DOI: 10.1021/ac9709920
(76) Haaland, D.M. Synthetic Multivariate Models to Accommodate Unmodeled Interfering Spectral Components during Quantitative Spectral Analyses. Appl. Spectrosc. 2000, 54 (2), 246–254.
(77) Chen, T.; Martin, E. The Impact of Temperature Variations on Spectroscopic Calibration Modelling: A Comparative Study. J. Chemom. 2007, 21 (5–6), 198–207. DOI: 10.1002/cem.1041
(78) Swierenga, H.; Wülfert, F.; de Noord, O.E.; de Weijer, A.P.; Smilde, A.K.; Buydens, L.M.C. Development of Robust Calibration Models in Near Infrared Spectrometric Applications. Anal. Chim. Acta. 2000, 411 (1), 121–135. DOI: 10.1016/S0003-2670(00)00718-2
(79) Wahl, P.R.; Treffer, D.; Mohr, S.; Roblegg, E.; Koscher, G.; Khinast, J.G. Inline Monitoring and a PAT Strategy for Pharmaceutical Hot Melt Extrusion. Int. J. Pharm. 2013, 455 (1), 159–168. DOI: 10.1016/j.ijpharm.2013.07.044
(80) Zeng–Ping, C.; Morris, J.; Martin, E. Extracting Chemical Information from Spectral Data with Multiplicative Light Scattering Effects by Optical Path-Length Estimation and Correction. Anal. Chem. 2006, 78 (22), 7674–7681. DOI: 10.1021/ac0610255
(81) Rinnan, A.; van den Berg, F.; Engelsen, S.B. Review of the Most Common Pre-Processing Techniques for Near-Infrared Spectra. TrAC – Trends Anal. Chem. 2009, 28 (10), 1201–1222. DOI: 10.1016/j.trac.2009.07.007
(82) Besseling, R.; Damen, M.; Tran, T.; Nguyen, T.; van den Dries, K.; Oostra, W.; Gerich, A. An Efficient, Maintenance Free and Approved Method for Spectroscopic Control and Monitoring of Blend Uniformity: The Moving F-Test. J. Pharm. Biomed. Anal. 2015, 114, 471–481. DOI: 10.1016/j.jpba.2015.06.019
(83) Andre, S.; Lagresle, S.; Hannas, Z.; Calvosa, E.; Duponchel, L. Mammalian Cell Culture Monitoring Using In Situ Spectroscopy: Is Your Method Really Optimised? Biotechnol. Prog. 2017, 33 (2), 308–316. DOI: 10.1002/btpr.2430
(84) Do, L.; Spencer, A.; Dost, F.; Farah, C. Oral Mucosal Lesions: Findings from the Australian National Survey of Adult Oral Health. Aust. Dent. J. 2014, 59 (1), 114–120. DOI: 10.1111/adj.12143
(85) Baronsky–Probst, J.; Moltgen, C.V.; Kessler, W.; Kessler, R.W. Process Design and Control of a Twin Screw Hot Melt Extrusion for Continuous Pharmaceutical Tamper-Resistant Tablet Production. Eur. J. Pharm. Sci. 2016, 87, 14–21. DOI: 10.1016/j.ejps.2015.09.010
(86) Pomerantsev, A.L.; Rodionova, O.Y. Process Analytical Technology: A Critical View of the Chemometricians. J. Chemom. 2012, 26 (6), 299–310. DOI: 10.1002/cem.2445
(87) Tu, J.V. Advantages and Disadvantages of Using Artificial Neural Networks Versus Logistic Regression for Predicting Medical Outcomes. J. Clin. Epidemiol. 1996, 49 (11), 1225–1231. DOI: 10.1016/S0895-4356(96)00002-9
(88) Martinez, L.; Peinado, A.; Liesum, L.; Betz, G. Use of Near-Infrared Spectroscopy to Quantify Drug Content on a Continuous Blending Process: Influence of Mass Flow and Rotation Speed Variations. Eur. J. Pharm. Biopharm. 2013, 84 (3), 606–615. DOI: 10.1016/j.ejpb.2013.01.016.
(89) Martinez, L.; Peinado, A.; Liesum, L. Inline Quantification of Two Active Ingredients in a Batch Blending Process by Near-Infrared Spectroscopy: Influence of Physical Presentation of the Sample. Int. J. Pharm. 2013, 451 (1), 67–75. DOI: 10.1016/j.ijpharm.2013.04.078
(90) Yang, Y.; Wang, L.; Wu, Y.; Liu, X.; Bi, Y.; Xiao, W.; Chen, Y. On-Line Monitoring of Extraction Process of Flos Lonicerae Japonicae Using Near Infrared Spectroscopy Combined with Synergy Interval PLS and Genetic Algorithm. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 182 (5), 73–80. DOI: 10.1016/j.saa.2017.04.004.
(91) Durao, P.; Fauteux-Lefebvre, C.; Guay, J.M.; Abatzoglou, N.; Gosselin, R. Using Multiple Process Analytical Technology Probes to Monitor Multivitamin Blends in a Tableting Feed Frame. Talanta 2017, 164 (3), 7–15. DOI: 10.1016/j.talanta.2016.11.013.
(92) Tanaka, R.; Takahashi, N., Nakamura, Y.; Hattori, Y.; Ashizawa, K.; Otsuka, M. In-Line and Real-Time Monitoring of Resonant Acoustic Mixing by Near-infrared Spectroscopy Combined with Chemometric Technology for Process Analytical Technology Applications in Pharmaceutical Powder Blending Systems. Anal. Sci. 2017, 33 (1), 41–46. DOI: 10.2116/analsci.33.41
(93) Mercier, S.M.; Rouel, P.M.; Lebrun, P.; Diepenbroek, B.; Wijffels, R.H.; Streefland, Process Analytical Technology Tools for Perfusion Cell Culture. M. Eng. Life Sci. 2016, 16 (1), 25–35. DOI: 10.1002/elsc.201500035
(94) Li, K.; Wang, W.; Liu, Y.; Jiang, S.; Huang, G.; Ye, L. Near-Infrared Spectroscopy as a Process Analytical Technology Tool for Monitoring the Parching Process of Traditional Chinese Medicine Based on Two Kinds of Chemical Indicators. Pharmacogn. Mag. 2017, 13 (50), 332.
(95) Wood, C.; Alwati, A.; Halsey, S.; Gough, T.; Brown, E.; Kelly, A. Paradkar, A. Near Infrared Spectroscopy as a Multivariate Process Analytical Tool for Predicting Pharmaceutical Co-Crystal Concentration, J. Pharm. Biomed. Anal. 2016, 129, 172–181. DOI: 10.1016/j.jpba.2016.06.010
(96) Schaefer, C.; Lecomte, C.; Clicq, D.; Merschaert, A.; Norrant, E.; Fotiadu, F. On-Line Near Infrared Spectroscopy as a Process Analytical Technology (PAT) Tool to Control an Industrial Seeded API Crystallization. J. Pharm. Biomed. Anal. 2013, 83 (9), 194–201 (2013). DOI: 10.1016/j.jpba.2013.05.015
(97) Schaefer, C.; Clicq, D.; Lecomte, C.; Merschaert, A.; Norrant, E.; Fotiadu, F. A Process Analytical Technology (PAT) Approach to Control a New API Manufacturing Process: Development, Validation and Implementation. Talanta 2014, 120, 114–125. DOI: 10.1016/j.talanta.2013.11.072
(98) Saerens, L.; Segher, N.; Vervaet, C.; Remon, J.P.; De Beer, T. Validation of an In-Line Raman Spectroscopic Method for Continuous Active Pharmaceutical Ingredient Quantification During Pharmaceutical Hot-Melt Extrusion. Anal. Chim. Acta 2014, 806, 180–187. DOI: 10.1016/j.aca.2013.11.020
(99) Saerens, L.; Ghanam, D.; Raemdonck, C.; Francois, K.; Manz, J.; Krüger, R.; Krüger, S.; Vervaet, C.; Remon, J.P.; De Beer, T. In-Line Solid State Prediction During Pharmaceutical Hot-Melt Extrusion in a 12 mm Twin Screw Extruder Using Raman Spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87 (3), 606–615. DOI: 10.1016/j.ejpb.2014.03.002
(100) Hisazumi, J.; Kleinebudde, P. In-Line Monitoring of Multi-Layered Film-Coating on Pellets Using Raman Spectroscopy by MCR and PLS Analyses. Eur. J. Pharm. Biopharm. 2017, 114 (5), 194–201 DOI: 10.1016/j.ejpb.2017.01.017
(101) Barimani, S.; Kleinebudde, P. Evaluation of In-Line Raman Data for End-Point Determination of a Coating Process: Comparison of Science-Based Calibration, PLS-Regression and Univariate Data Analysis. Eur. J. Pharm. Biopharm. 2017, 119 (10), 28-35. DOI: 10.1016/j.ejpb.2017.05.011
(102) Kandpal, L.M.; Tewari, J.; Gopinathan, N.; Boulas, P.; Cho, B.K. In-Process Control Assay of Pharmaceutical Microtablets Using Hyperspectral Imaging Coupled with Multivariate Analysis. Anal. Chem. 2016, 88 (22), 11055. DOI: 10.1021/acs.analchem.6b02969
(103) Da Silva, V.H.; Vieira, F.S.; Rohwedder, J.J.; Pasquini, C.; Pereira, C.F. Multivariate Quantification of Mebendazole Polymorphs by Terahertz Time Domain Spectroscopy (THZ-TDS). Analyst 2017, 142 (9) 1519–1524. DOI: 10.1039/C6AN02540D
(104) Peters, J.; Taute, W.; Bartscher, K.; et al. Design, Development and Method Validation of a Novel Multi-Resonance Microwave Sensor for Moisture Measurement. Anal. Chim. Acta 2017, 961 (4), 119–127. DOI: 10.1016/j.aca.2017.01.021
Faten Farouk is with the Department of Pharmaceutical Chemistry at Ahram Canadian University in Cairo, Egypt. Rania M. Hathout is with the Department of Pharmaceutics and Industrial Pharmacy at Ain Shams University, in Cairo, Egypt. Ehab F. Elkady is with the Faculty of Pharmacy at Cairo University, in Cairo, Egypt. Direct correspondence to: ehabelkady75@gmail.com ●

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.