Infrared Spectroscopy of Polymers, XI: Introduction to Organic Nitrogen Polymers
Until now, our discussion of polymers has been limited to functional groups containing carbon, hydrogen, and oxygen. In this column, we begin covering polymers that have functional groups containing nitrogen as well. We review the structures and bonding characteristics of the nitrogen atom, and then begin our examination of the spectra of organic nitrogen polymers with a very important class of polymers: polyamides.
In the last column, we concluded our study of the infrared (IR) spectroscopy of carbonyl-containing polymers by discussing acrylates (1). Traditionally, the chemical element nitrogen is considered part of organic chemistry (2). In this column and several following it, we turn our attention to polymers that contain organic nitrogen compounds.
The nitrogen atom has an atomic number of seven and contains five outer shell electrons. The drive of chemical elements, of course, is to get their outer shells filled. In most organic compounds, nitrogen tries to fill its outer shell by forming three chemical bonds to other atoms, be they hydrogens, carbons, or whatever. Carbon and nitrogen can form single, double, or triple bonds, as seen in Figure 1.
FIGURE 1: Examples of nitrogen-carbon bonds. From left to right, a carbon nitrogen single bond, double bond, and triple bond.
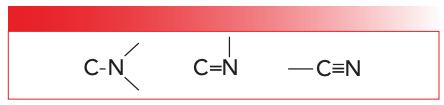
Note for the C-N bond seen on the left in Figure 1 that the bond angles are approximately 120°, and there are three atoms bonded to the nitrogen. For the C=N bond seen in the middle of Figure 1, the bond angles are approximately 90° (2), and there are two atoms attached to the nitrogen. Lastly, for the C≡N bond seen on the right in Figure 1, the bond angles are approximately 180° and there is one atom (carbon) attached to the nitrogen. Nitrogen has an electronegativity of three, which means it tends to hog the electrons in any bonds it forms. Carbon and hydrogen have electronegativities of 2.5 and 2.1, respectively (2), so C-N and N-H bonds are covalent.
Since we need to study nitrogen containing polymers, is there an IR signature for the presence of nitrogen in a sample? This question was answered previously when we first studied nitrogen containing functional groups (3). Briefly, C-N stretching vibration peaks are common but weak because they have a small value of dμ/dx, which is the change in dipole moment with respect to bond length during a vibration. Recall (4) that the intensity of the IR peaks depends upon (dμ/dx)2 amongst other things. The small value of (dμ/dx)2 means C-N stretching peaks are weak and hard to see. Also, they show up in the middle of the fingerprint region from 1400 to 1000 cm-1 (all peak positions and ranges will be in cm-1 units going forward even if not so noted) where they are lost in the level of detail there. Thus, C-N stretches are not good group wavenumbers and are not useful for determining if a sample contains nitrogen.
C=N stretching peaks are even more problematic because this functional group is not stable and rarely seen. Lastly, the C≡N or nitrile group has a strong and unique peak around 2200 as we have seen (5). This peak is an excellent group wavenumber, but not all nitrogen containing polymers have a C≡N bond, so this peak cannot be used as a diagnostic marker for the presence of nitrogen in a polymer. This means we have struck out with trying to use carbon-nitrogen stretching vibrations to solve our problem. As it turns out, N-H stretching and bending peaks are our best bet for figuring out whether there is nitrogen in a polymer (3).
Polyamides
FIGURE 2: The structural framework of the amide functional group.
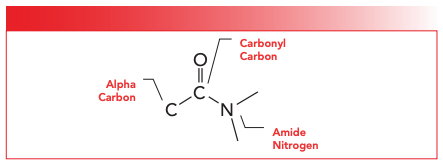
The first type of nitrogen containing compounds we will discuss are polyamides. These polymers contain an amide group in their backbone. The structure and parts of the amide functional group are seen in Figure 2. There are three types of amides: primary; secondary; and tertiary. These amides are named depending upon the number of C-N bonds present in the functional group as you can see in Figure 3. Note that as the number of C-N bonds goes up, the number of N-H bonds goes down. Thus, primary amides have one C-N bond and two N-H bonds; secondary amides have two C-N bonds and one N-H bond; and tertiary amides have three C-N bonds and no N-H bonds. Polyamides can be divided into roughly three groups. Nylons, which you have probably heard of, is another name for polyamides (6). Aromatic polyamides, where there is an aromatic ring in the polymer backbone, are also known as aramids (7). Lastly, an important group of polyamides are proteins, which I am sure you have heard of. Now, biochemists hate it when I say this, but from the point of view of an organic chemist, proteins are polymers made from amino acid monomers where each repeat unit is linked to the next via an amide bond. We will discuss the spectroscopy of proteins in a later column.
FIGURE 3: The structural frameworks of primary, secondary, and tertiary amides.
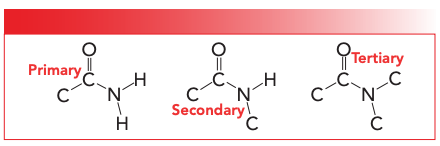
As we have studied previously (8), all amides have their C=O stretching peaks between 1680 and 1630 because all of them are conjugated. Because most polyamides contain secondary amide linkages, that will be our sole focus in the study of polyamides. Table I lists the group wavenumbers for secondary amides.
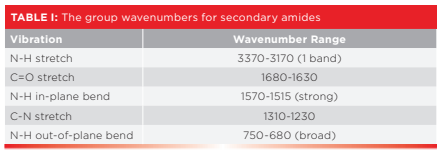
The number of N-H stretching peaks for amides equals the number of N-H bonds. Thus, for secondary amides there is only one N-H stretching peak which falls from 3370 to 3170 as stated in Table I. This peak is at 3301 and is labeled A in the spectrum of nylon 6,6 seen in Figure 4.
FIGURE 4: The structure and infrared spectrum of nylon 6,6.
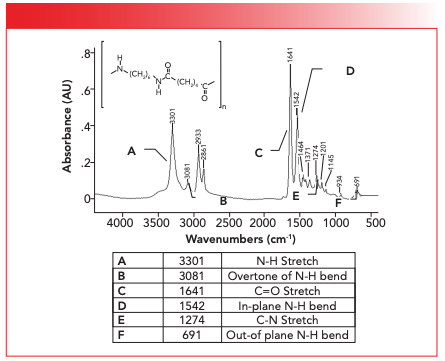
Note that the N-H stretching peak in Figure 4 falls in the same range as O-H stretches (9). However, N-H stretches are weaker than O-H stretches because dμ/dx is greater for O-H than N-H bonds. Also, N-H stretches are narrower than O-H stretches because hydrogen bonding is weaker for N-H bonds than O-H bonds (10). The C=O stretch for nylon 6,6 falls at 1641 as expected from Table I, and is labeled C in Figure 4. Note that it has an intense peak next to it labeled D at 1542, which is assigned as the N-H in plane bend (10). In the many years I have been writing these columns, we have said very little about in-plane bending vibrations because they are often times weak and show up in the crowded fingerprint region where they are hard to see. However, the N-H stretch of secondary amides are unusually intense, making them excellent group wavenumbers. Also, the N-H stretch of secondary amides is one of the few sharp, intense peaks we see in the 1600 to 1500 range in IR spectroscopy (10).
Note that in Figure 4 the C=O stretch and N-H in-plane bend fall at about 1640 and 1540 respectively, and that they are the two biggest peaks in the spectrum. In my experience, if I see the spectrum of a polymeric sample with a pair of intense peaks near 1640 and 1540, my first thought is nylon.
The C-N stretch for nylon 6,6 is at 1274 and is labeled E in Figure 4. As I mentioned above, C-N stretches are weak and peak E in the figure is a perfect example of this. Notice there are a lot of other peaks around it. If this fingerprint region were any busier we might not see the C-N stretching peak at all. Lastly, the N-H wag for nylon 6,6 falls at 691 and is labeled F in Figure 4. This peak is small but is noticeably broadened because of hydrogen bonding (10).
Nylon 6,6 versus Nylon 6
In the nomenclature for nylons, the numbers refer to the number of carbon atoms in the monomer(s) used to make the polymer. For example, for nylon 6,6, each repeat unit contains six carbon atoms. There is a variant of nylon 6,6 called nylon 6. The structures of these two polymers are seen in Figure 5.
FIGURE 5: A comparison of the structures and spectra of nylon 6,6 and nylon 6 in the 1350 to 1050 cm-1 region.
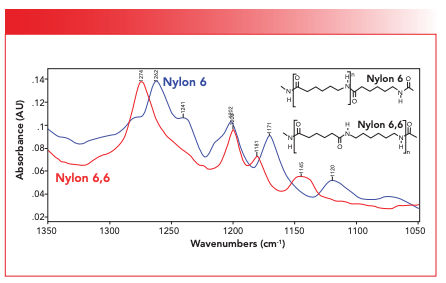
Note that for nylon 6,6 the order of functional groups is C=O, C=O, N-H, and N-H, whereas for nylon 6, it is C=O, N-H, C=O, and N-H. This is a small but real chemical difference. Now, both nylon 6,6 and nylon 6 are important commercially, and both these polymers are made into fibers for clothing and carpets. It would be useful if IR spectroscopy could tell the difference between the two. Figure 5 shows the spectra of these two polymers in part of the fingerprint region from 1350 to 1050. Note that the C-N stretch of nylon 6,6 is at 1274, whereas for nylon 6, it is at 1262. Also, nylon 6 has a peak at 1171 that nylon 6,6 does not have, and nylon 6,6 has a peak at 1145 that nylon 6 does not have. The point is that even though these two polymers have very similar chemical structures, they have spectra that can be distinguished from each other. This has been important in my own work when I once was tasked with developing a method for distinguishing nylon 6,6 from nylon 6 so these materials could be sorted and recycled separately. Infrared spectroscopy did the trick (11). This then is an example of the utility of IR spectroscopy to distinguish similar materials from each other.
Conclusions
We reviewed the topic of nitrogen containing functional groups, and then looked at the spectrum of a polyamide, nylon 6,6, in detail. It shows all the classic peaks of a secondary amide, and the 1640/1540 intense pair of peaks from the C=O stretch and N-H in-plane bend, which are a strong sign that a polymeric material is a nylon. We wrapped up by showing how infrared spectroscopy can easily distinguish between two chemically similar and economically important polymers, nylon 6,6 and nylon 6.
References
(1) Smith, B. C. Infrared Spectroscopy of Polymers X: Polyacrylates, Spectroscopy 2023, 38 (1), 10–14. https://doi.org/10.56530/spectroscopy.mi9381w4
(2) Stretiweiser, A. and Heathcock, C. Introduction to Organic Chemistry; Macmillan, 1976.
(3) Smith, B. C. Organic Nitrogen Compounds, Part I: Introduction. Spectroscopy 2019, 34 (1), 10–15.
(4) Smith, B. C. Why Spectral Interpretation Needs to Be Taught. Spectroscopy 2015, 30 (1), 16–23.
(5) Smith, B. C. Organic Nitrogen Compounds IV: Nitriles. Spectroscopy 2019, 34 (1), 18–21, 44.
(6) Wikipedia, Nylon (accessed February 2023). https://en.wikipedia.org/wiki/Nylon.
(7) Wikipedia, Aramid (accessed February 2023). https://en.wikipedia.org/wiki/Aramid.
(8) Smith, B. C. Organic Nitrogen Compounds VI: Introduction to Amides. Spectroscopy 2019, 34 (11), 30–33.
(9) Smith, B. C. The C-O Bond, Part I: Introduction and the Infrared Spectroscopy of Alcohols. Spectroscopy 2017, 32 (1), 14–21.
(10) Smith, B. C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press, 1999.
(11) Smith, B. C. Unpublished Results.
Brian C. Smith, PhD, is the founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer-reviewed paper, and has written three books on spectroscopy. As a trainer, he has helped thousands of people around theworld improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at SpectroscopyEdit@MMHGroup.com.


AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).
New Study Provides Insights into Chiral Smectic Phases
March 31st 2025Researchers from the Institute of Nuclear Physics Polish Academy of Sciences have unveiled new insights into the molecular arrangement of the 7HH6 compound’s smectic phases using X-ray diffraction (XRD) and infrared (IR) spectroscopy.