The Role of Triple Quadrupole GC–MS in Steroid Analysis
Special Issues
The authors discuss the use of GC-MS in drug doping testing.
Drug doping testing requires methods that are highly sensitive, specific, and quantitative, particularly for steroid analysis. The demand for such methods will only increase in the future, and technologies must continue to evolve. Gas chromatography–triple quadrupole mass spectrometry (GC–QQQ) meets this challenge by providing doping control laboratories with the ability to do screening, confirmation, and quantification in one analysis, in complex sample matrices, without the need to change their current extraction and GC separation methods.
The recent Beijing Olympic Games reminded us of the value of international sport competition for fostering peace and mutual understanding worldwide. Unfortunately, we are also reminded that there are those athletes who will sacrifice their careers and violate the trust of their countrymen by using performance-enhancing drugs to give them a competitive edge, and cheating is as old as the Olympic Games.
The use of performance-enhancing drugs in sporting events, either in the form of supplements or highly purified and potent compounds, eventually resulted in the establishment of systematic screening protocols to detect and prevent the use of such substances. This practice of chemical enhancement of athletic performance is commonly referred to as "doping." The death of British cyclist Tommy Simpson in 1967 from an amphetamine-induced heart attack sparked the introduction of routine, in-competition drug testing by most international sports federations, and in 1972 it made its debut at the Olympic Games in Munich. After the formation of the medical committee of the International Olympic Committee (IOC) in 1967, a list of prohibited substances was established. The list is updated frequently and supervised by the World Anti-Doping Agency (WADA), which was formed in 2000. The list currently includes five categories of prohibited substances (anabolic agents, hormones and related substances, beta-agonists, anti-estrogenic agents, and diuretics and other masking agents); three prohibited methods (enhancement of oxygen transfer, chemical or physical manipulation of samples, and gene doping); and four categories of substances prohibited only during competition (stimulants, narcotics, cannabinoids, and glucocorticosteroids). A minimum required performance limit (MRPL) is established for each prohibited compound, examples of which are shown in Table I.
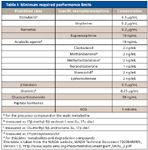
Table I: Minimum required performance limits
WADA-accredited laboratories are required to demonstrate that they can routinely perform screening and confirmatory analyses at the MRPL established for each prohibited compound, following general guidelines prescribed by WADA. Procedures to account for the integrity of each specimen and preserve the chain of custody are in place to track handling and storage from the point of collection to final disposal of the specimen. These laboratories are responsible for assuring chain of custody by recording receipt of the samples, assuring proper handling and storage through intralaboratory identification and tracking, and ensuring confidentiality. The testing is then performed, the data archived, and results communicated observing the same strict protocols governing chain of custody. Ongoing original research and method refinement activities are also required of WADA-accredited laboratories to optimize performance and develop even more effective methods to identify new prohibited substances. Consequently, the methods and analytical technologies of these laboratories are constantly evolving.
GC–MS and LC–MS Are the Testing Platforms of Choice
WADA-accredited laboratories use methodologies that have been optimized to screen large numbers of athlete samples (usually urine) for the presence of prohibited substances, then confirm any presumptive positive result with an orthogonal method that is both highly selective and unequivocally confirmatory. The majority of standard protocols used in doping control laboratories are based on an initial chromatographic separation of the complex urine extract using either gas chromatography (GC) or high performance liquid chromatography (HPLC), followed generally by mass spectrometry (MS) analysis of the chromatographic eluent to detect and quantify the vast range of prohibited substances. GC is suitable for compounds that can be vaporized easily without degrading or decomposing the compound, and was the first chromatographic method to be used extensively in doping control laboratories. HPLC is more suitable for thermolabile and highly polar compounds that cannot be readily vaporized, and for separation of large biomolecules such as proteins and peptides.
The combination of GC or HPLC with MS provides a highly sensitive, selective, and versatile tool for the detection of WADA-prohibited compounds. A wide range of MS analyzers is available, each having advantages for particular applications. The most common type of MS analyzer used in doping analysis is the single-quadrupole MS system, which can be used in either the full-scan or selected ion monitoring (SIM) mode in screening protocols. Mass spectrometers with more than one mass analyzer (tandem MS or MS-MS systems) provide the next higher level of confidence in the identification of banned substances. These instruments first isolate the precursor ion or other intense fragment ion present in the full-scan MS spectrum and then fragment this precursor target ion into smaller product ions. The resulting product ion mass spectrum can be compared with that obtained from an authentic standard (or interpreted a priori if no standard is available) as part of the identity confirmation process. The ion trap MS-MS system (single analyzer, tandem-in-time operation) has found utility in many doping control laboratoriess for screening due to its ability to provide both MS spectral and MS-MS product ion spectral data, and in particular its sensitivity for acquiring product ion spectra. This performance feature means that the ion-trap mass analyzer can be used for analyte confirmation in selected circumstances, but co-eluted substances with isobaric precursor masses can result in a complex product ion spectrum of questionable value for confirmation. The tandem quadrupole mass spectrometer (sometimes called a "triple quadrupole" or "QQQ" system) is the definitive tool for high-confidence screening of target analytes, their confirmation, and quantification of these prohibited compounds in a single GC–MS-MS analysis.
GC was the first analytical technique used to detect prohibited substances in athletes, and GC–MS has become the workhorse of doping control laboratories worldwide. It is routinely used to test for anabolic agents as well as beta-2 agonists, anti-estrogens, diuretics, stimulants, narcotics, cannabinoids, and beta-blockers (1). Screening for these compounds is performed using either the full-scan mode or the selected ion monitoring (SIM) mode with a single quadrupole mass analyzer. SIM is also used for confirmation of a suspect positive by determining the relative ratios of multiple ions being monitored for each target compound and comparing these to ratios obtained for authentic standards. GC–MS-MS with an ion trap mass analyzer is used in a number of doping control laboratories as well, to provide added sensitivity and specificity. The recent availability of GC–QQQ systems promises to extend the capability of drug doping laboratories by providing the ability to screen, confirm, and quantify prohibited compounds in one run, even in complex matrices.
GC–QQQ for Steroid Analysis
Doping control efforts focus to a significant extent on anabolic steroids, as they are probably the most widely abused category of prohibited substances. The earliest drug doping test methods developed were for steroid detection, resulting for example in the confirmation of stanozolol in a urine sample from sprinter Ben Johnson in the1988 Olympic Games, with the subsequent forfeiture of his gold medal and two world records. More recently, the detection of an elevated testosterone/epitestosterone (T/E) ratio led to the stripping of the 2006 Tour de France title from Floyd Landis, and the imposition of a two-year ban from sanctioned competition. The analysis of this group of compounds also receives close attention in the laboratory, as they must be detected at such low levels (Table I), requiring ongoing development of more highly selective and sensitive testing methods.
Many doping control laboratories have traditionally used high resolution mass spectrometry (HRMS) or GC–ion trap MS-MS to meet the challenge of identifying and quantifying very low levels of very similar steroid compounds. However, HRMS is a technology that is expensive to acquire and requires a great deal of training and expertise to operate. Ion-trap MS-MS is a more economical option and somewhat easier to operate, but for some analytes, it may require the use of MS-MS-MS to achieve the requisite specificity, potentially resulting in a failure to achieve required sensitivity. On the other hand, the steroid detection and quantification requirements of the doping control laboratory are an excellent fit for the capabilities of the GC–QQQ platform. Selected reaction monitoring (SRM) with a QQQ system provides detection of trace amounts of target compounds in complex matrices, providing screening, confirmation, and quantification in one analysis. The precursor ion of interest generated by the source is selected and isolated in the first (Q1) quadrupole. A hexapole collision cell (Q2) fragments the selected ion, and the product ions are selected in Q3. By choosing product ions from this transition that are characteristic for the steroids of interest, chemical noise is separated from the signal, providing very high sensitivity and selectivity, even in very dirty matrices. With careful selection of a unique precursor to product ion transition for each steroid, it is practical to quickly and easily create SRM assay methods that can be used to identify and quantify simultaneously many of the prohibited steroids in a sample.
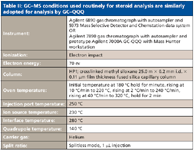
Table II: GCâMS conditions used routinely for steroid analysis are similarly adopted for analysis by GCâQQQ
High Sensitivity of Detection
Doping control laboratories must be able to detect 2 ppb or less of each of the following steroids: clenbuterol, 19-norandrosterone, 17α-methyl-5β-androstane-3α, 17β-diol, epimetendiol (methandienone), and 3'-hydroxystanozolol (stanozolol). Methods have been developed previously in certain doping laboratories, utilizing the GC–ion trap MS-MS platform, that have demonstrated the potential to screen and confirm less than 2 ppb each of these compounds in a low-level quality control (LQC) standard. This is generally accomplished by examining the extracted ion current profiles of the target ions present in MS spectra or in MS-MS (or MS-MS-MS) product ion spectra. However, many laboratories conduct routine screening analysis of steroids in urine using GC–MS operating in the SIM mode. These laboratories can use the same chromatographic separation protocols to detect and confirm as many as 120 analytes in one run using SRM on a GC–QQQ system (Table II). This includes the components of the LQC standard, which can have the same GC retention times on a GC–QQQ system as they do on a GC–MS system.
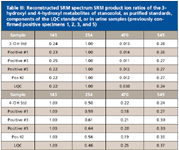
Table III: Reconstructed SRM spectrum SRM product ion ratios of the 3-hydroxyl and 4-hydroxyl metabolites of stanozolol, as purified standards, components of the LQC standard, or in urine samples (previously confirmed positive specimens 1, 2, 3, and 5)
Positive Identification — Anabolic Steroids
Using SRM on the GC–QQQ system, steroids can be reliably separated, detected, identified, and quantified. Figure 1 demonstrates separation and identification of the components in the LQC standard, all present at only 2 ppb. Very similar compounds such as the 3-hydroxystanozolol and 4-hydroxystanozolol metabolites of stanozolol can be identified and quantified, using the same four ion transitions with SRM, even though they have very similar retention times (Figure 2). The two derivatives give very different relative comparative abundances of the four selected product ions. The data demonstrate that peak height ratios of the product ions, versus a reference product ion, are characteristic of each metabolite, allowing positive identification of both. These ratios are very reproducible, whether using a standard containing only the 3-OH or 4-OH metabolite, the LQC standard, or positive urine samples containing various levels of the metabolites (Table III), allowing reliable identification.
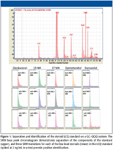
Figure 1
Endogenous Steroids
Doping control laboratories must be able to detect prohibited steroids against a background of several endogenous steroids whose levels must be accurately quantified. For this purpose, an endogenous quality control standard (EQC) with known quantities of endogenous steroids can be used to validate detection limits and screening efficacy. Two of the most significant components of this standard are testosterone and epitestosterone. Epitestosterone is an inactive stereoisomer of testosterone, and in an average adult male the ratio of the quantities of the two is about 1:1. If exogenous testosterone is being taken as a performance-enhancing drug, the T/E ratio increases. WADA has set a ratio of 4:1 as the threshold above which further investigation is required to confirm that the elevated level is the result of exogenous testosterone administration by the athlete. Accurate estimation of this ratio is dependent upon the ability to accurately identify and quantify each of these two steroids. Identification is accomplished by GC–QQQ SRM analysis of the two stereoisomers, providing nine transitions with which to quantify testosterone and epitestosterone. The peak height ratios of the extracted product ion profiles for three of the principal product ions from these transitions (for example, SRM transition 432 > 209; 432 > 301; 432 > 196) provide essentially the same ratios, giving accurate and reliable estimation of the T/E ratio (Figure 3).

Figure 2
Other endogenous steroid metabolites of testosterone including androsterone, etiocholanolone, dehydroepiandrosterone (DHEA), and 5α- and 5β-androstanediols may be quantified in a similar approach using various transitions for the quantification of these analytes.
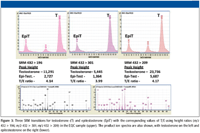
Figure 3
The sports community is moving toward the establishment of a "passport" for each athlete, based on the principle that biological monitoring throughout an athlete's career should make any illegal preparation harder to implement. Such monitoring will ensure that routine quantification of endogenous steroids becomes even more important. Basing this quantification on SIM acquisition techniques may result in inaccurate determinations of these levels, as SIM may be more prone to interferences from the matrix. The seven aforementioned endogenous steroids can indeed be reliably and reproducibly analyzed by SRM on a GC–QQQ instrument, paving the way to realizing the goal of closer scrutiny for athletes both during competition as well as out of competition. This athlete's passport then becomes a historical record of endogenous steroid levels, permitting any anomalies that may result from doping to be recognized quickly.
Conclusion
As long as athletes look to performance-enhancing drugs to get an edge on their competition, there will be a need for doping control laboratories that have an ever-growing arsenal of technologies and methodologies with which to detect current prohibited substances, as well as substances that may be developed in the future. The demand for sensitivity, specificity, and accurate quantification will only increase, and technologies must continue to evolve to meet the challenge. GC–QQQ is well-suited to meet this challenge because it provides doping control laboratories with the ability to increase sensitivity and specificity in complex matrices, with a modest investment in new equipment, and without the need to change their current extraction and GC separation methods.
Aishah Latiff is Director of the Doping Control Center in Penang, Malaysia. Melissa Churley is an Applications Chemist at Agilent Technologies, Santa Clara, California.
References
(1) C.K. Hatton, Pediatr. Clin. N. Am. 54, 713–733 (2007).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.