Spectrophotometric Determination of Thiosulfate in Desulfurization Solutions by Decoloration of Methylene Blue
This article proposes a method for the determination of thiosulfate by decoloring spectrophotometry with methylene blue as an oxidant. Methylene blue has redox properties. Its oxidized form is blue, and its reduced form is colorless. Thiosulfate is a medium-strength reducing agent. In an acidic medium, the two can undergo redox reactions to reduce methylene blue and cause the solution to exhibit a fading reaction within a certain range, and the degree of fading is proportional to the amount of thiosulfate added. This method measures the change in absorbance (AU) at a fixed wavelength of 664 nm, and ΔAU has a linear relationship with the content of thiosulfate. The linear range is 0–0.3 mmol/L. From this curve, the content of thiosulfate in the desulfurization solution can be accurately measured. This method is not interfered with by other secondary salts in the desulfurization solution during the thiosulfate determination process, and has high sensitivity. The color change can be observed by the naked eye to judge whether there is thiosulfate in the solution. This method is simple to perform and is an improved method for the determination of thiosulfate in a desulfurization solution.
Coking plant chemical gas will inevitably have side reactions during desulfurization, generating SCN-, S2O32-, SO32-, SO42-, and other acid ions (1–3) during the recycling of the desulfurization liquid, and cause the accumulation of by-product salt, leading to problems such as low desulfurization efficiency and large waste liquid discharge pollution. These side reactions cause serious harm to the desulfurization process (4). At the same time, the side reactions also cause serious harm to the desulfurization production system. During the production process, a part of the desulfurization liquid needs to be discharged, while the new desulfurization liquid is added. The amount of discharge increases the production cost and causes environmental concerns (5,6). Therefore, the proper treatment of desulfurization liquid is of economic and environmental significance at multiple levels.
Thiosulfate is one of the secondary salts produced in the desulfurization process. In order to ensure the stable operation of the desulfurization system in the production process, the content of thiosulfate must be controlled within an appropriate range. In the production process, in order to meet the requirements of production regulation, the accuracy and timeliness of detection is particularly important. At present, the commonly-used methods for the determination of thiosulfate are high performance liquid chromatography (7,8), ultraviolet spectrophotometry (9), and the iodometric method (10,11). However, the iodine standard solution is unstable and needs to be calibrated regularly, it is necessary to eliminate interfering substances before the measurement, and the measurement process is complicated, time-consuming, and lacks sensitivity.
In the current work, the redox reaction between methylene blue and thiosulfate causes the methylene blue to fade; therefore, a colorimetric-photometric method was developed to sensitively determine thiosulfate concentration, and the practicability of the method was proven by measur- ing the content of thiosulfate in the desulfurization solution. Compared with previous methods, this method has the advantages of strong anti-interference ability, simple operation, and high sensitivity.
Theory
Methylene blue has redox properties, its oxidized form is blue, and its reduced form is colorless (12). Thiosulfate is a medium-strength reducing agent (13); in an acidic medium, the two can undergo a redox reaction, reducing methylene blue and causing the solution to undergo a fading reaction within a certain range. The degree of fading is proportional to the amount of thiosulfate added; based on this reaction principle, a working curve is obtained by plotting ΔAU as the ordinate and the concentration of thiosulfate as the abscissa. From this curve, the content of thiosulfate in the desulfurization solution can be accurately determined.
Experimental
Instruments and Reagents
- UV5 ultraviolet-visible spectrophotometer
- Standard solution of sodium thiosulfate: 0.01019 mol/L (Shandong Academy of Metallurgical Sciences).
- Methylene blue solution: 0.2500 g/L; sulfuric acid solution: 1 mol/L.
Detection Method of Thiosulfate
- Take two test tubes and add 1.5 mL of 1 mol/L sulfuric acid solution and 0.35 mL of 0.25 g/L methylene blue solution
- Add a certain amount of 0.01 mol/L sodium thiosulfate standard solution to one of them, and dilute the two test tubes to a final total volume of 10 mL with DI water.
The reaction was carried out at room temperature for 20 min, and water was used as the diluent. The absorbances AU0 and AU of the two solutions were measured at 664 nm, and the value of ΔAU was calculated.
Pretreatment of Real Samples
Desulfurization liquid samples are provided by Coking Plant of SD Steel Rizhao Co., Ltd. The sample was subjected to a simple filtering process, diluted tenfold with DI-water for further analysis.
Results and Discussion
Absorption Spectrum
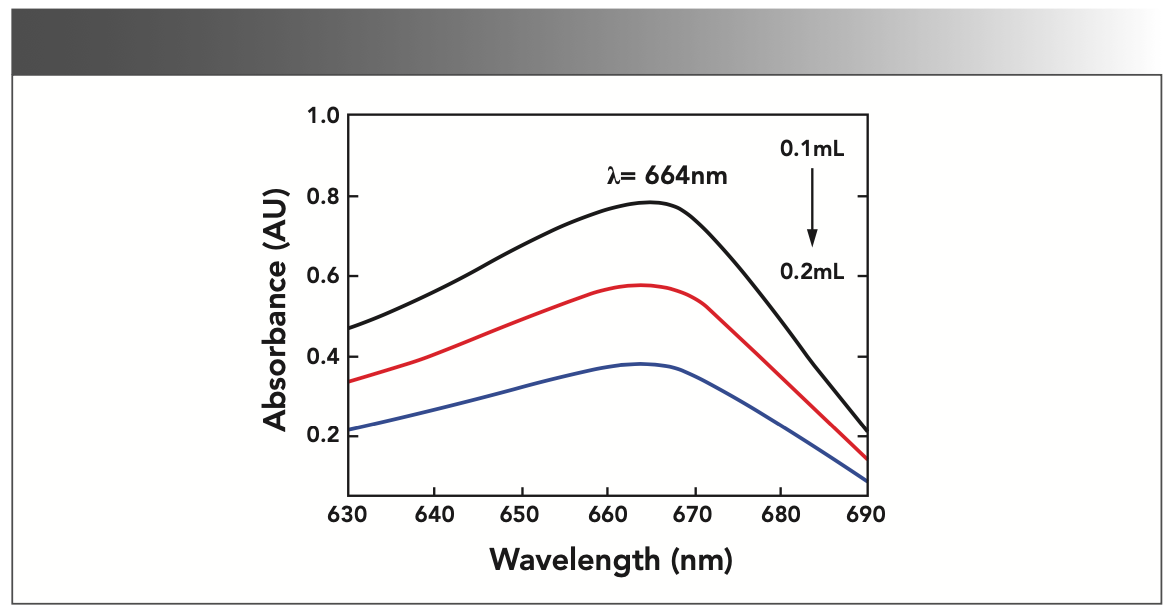
The ΔAU were calculated for 0.0 mL (black); 0.1 mL, 0.01 mol/L (red); and 0.2 mL, 0.02 mol/L (blue) sodium thiosulfate. The ΔAU = AU0–AU, was calculated and the ΔAU curve was plotted, as shown in Figure 1. It can be seen that the maximum absorption wavelength of the blank solution and the solution containing sodium thiosulfate is at 664 nm, and the ΔAU value at this point is the largest—that is to say, the fading effect of sodium thiosulfate on methylene blue is the most significant, so the wavelength of 664 nm was selected as the measurement wavelength.
FIGURE 1: Graph of absorbance (AU) spectrum with change in sodium thiosulfate.
Effects of Acidic Medium Dosage
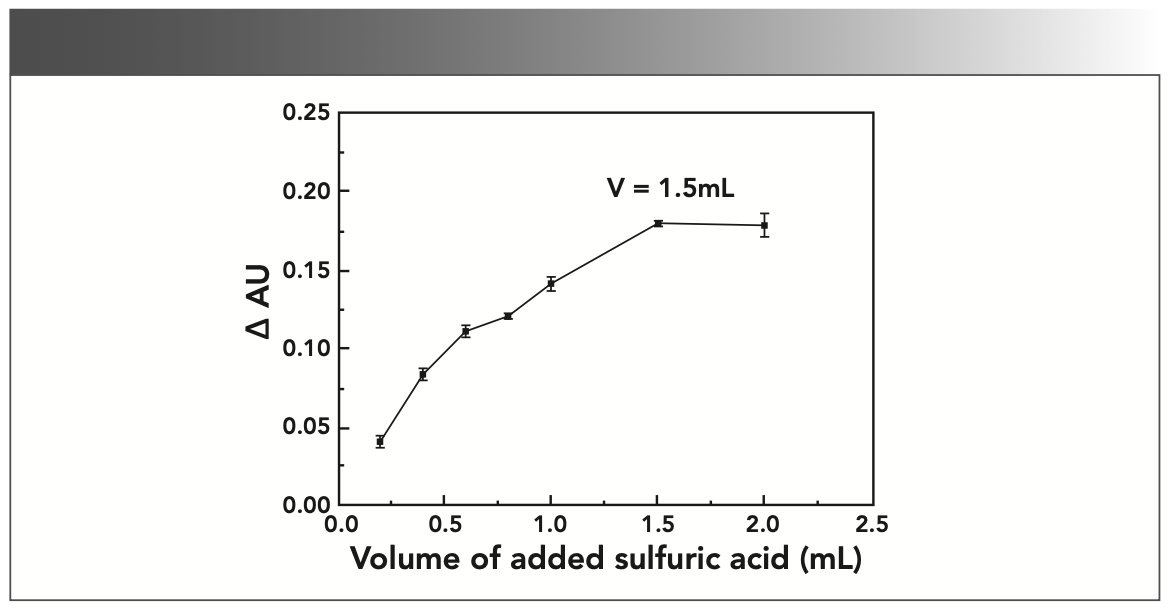
In this experiment, sulfuric acid was selected as the acidic medium, and the amount of sulfuric acid was optimized. The experimental results show that when the amount of 1 mol/L H2SO4 corresponding to 1.5 mL is added, the difference ΔAU of the absorbance of the solution is the largest and tends to be stable, as shown in Figure 2; thus, 1.5 mL 1 mol/L H2SO4 was selected as the optimum reaction medium in this experiment.
FIGURE 2: Effect of sulfuric acid addition on absorbance (AU).
Effect of Methylene Blue Dosage
As an important substance in the reaction system, the amount of methylene blue may affect the measurement results, so the amount of methylene blue is optimized. The experimental results show that when the amount of 0.2500 g/L methylene blue (0.35 mL) is present, the difference ΔAU of the absorbance of the solution is the largest. Therefore, 0.2500 g/L methylene blue is used as the reaction amount in this experiment.
Effect of Reaction Temperature
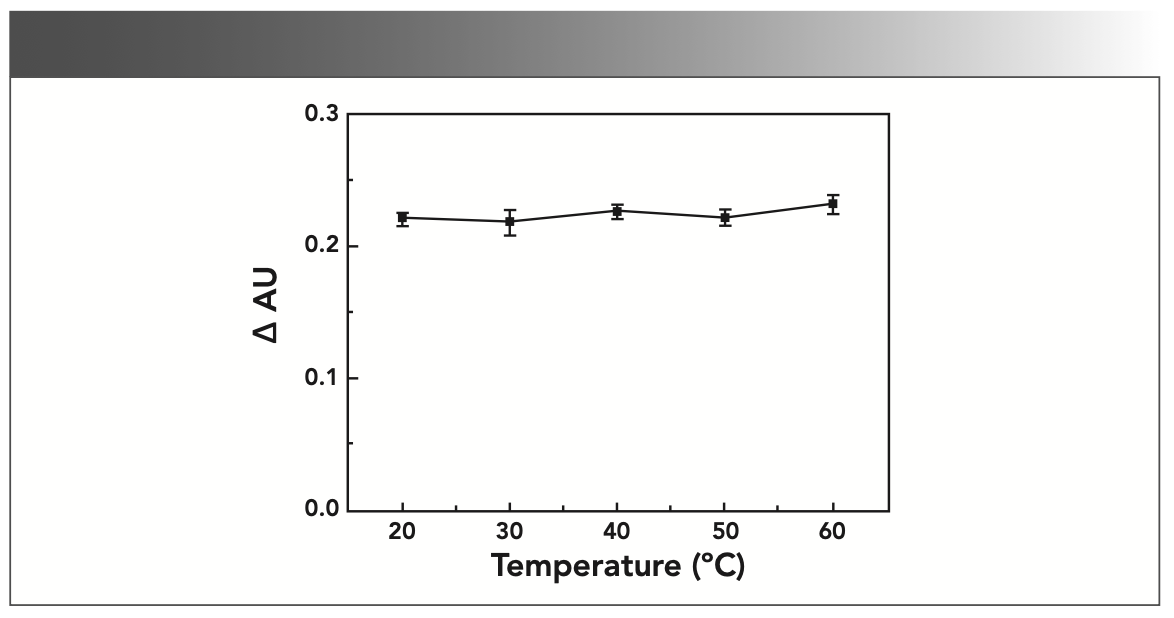
The experiment results show that, when the reaction temperature is different, the difference ΔAU of the absorbance of the solution has almost no effect, as shown in Figure 3. This experiment was chosen to measure at room temperature.
FIGURE 3: Effect of reaction temperature on absorbance (AU).
Effects of Reaction Time
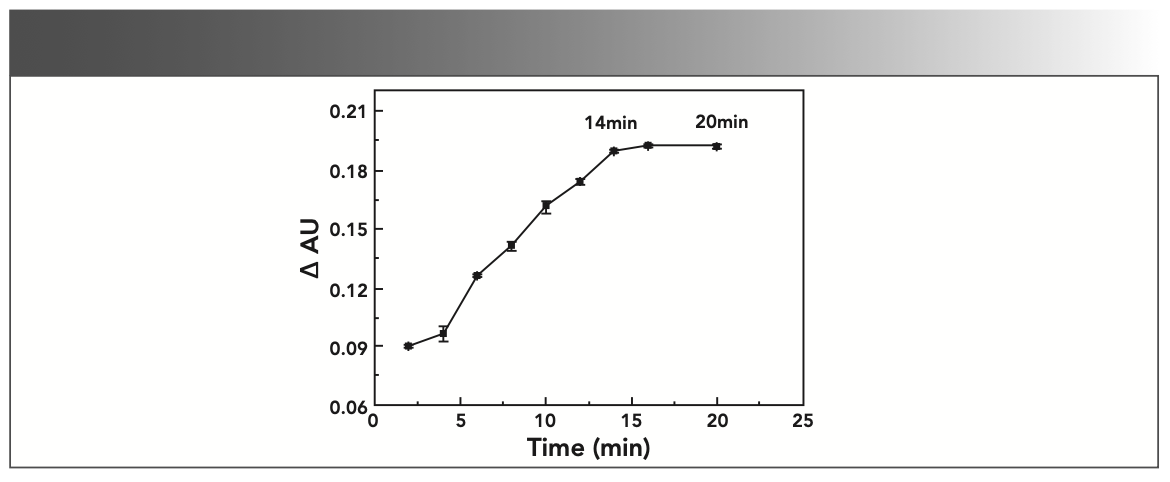
The experiment results show that when the reaction time is in the range of 1–20 min, the difference ΔAU of the absorbance has a maximum value and tends to be stable, as shown in Figure 4. In order to ensure that the reaction is complete, the reaction time is set to 20 min.
FIGURE 4: Effect of reaction time on absorbance (AU).
Working Curve
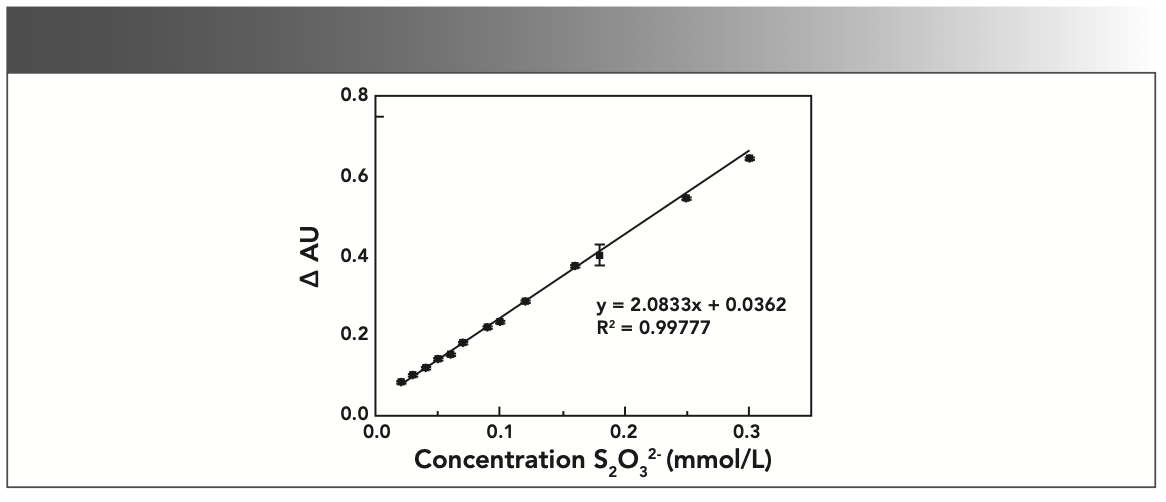
Under the selected optimized conditions, different amounts of 0.01 mol/L Na2S2O3 (sodium thiosulfate) standard solution were added to determine the absorbance, and a standard curve was drawn, as shown in Figure 5. The results show that when the concentration of S2O32- is in the range of 0–0.3 mmol/L, the concentration of S2O32- and ΔAU have a good linear relationship. The standard curve regression equation is ΔAU = 2.0833C + 0.0362, and the coefficient of determination, R2 = 0.99777.
FIGURE 5: Standard curve of concentration (S2O32–) vs. change in absorbance (AU).
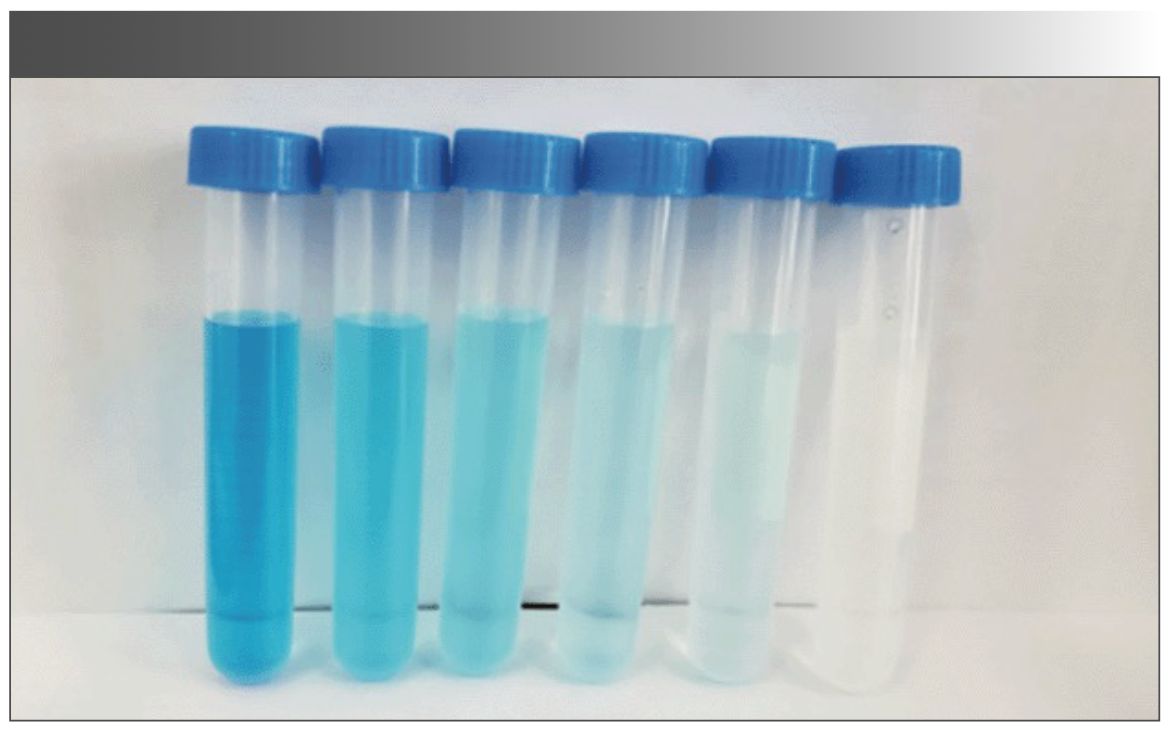
It can be clearly seen from the color that, as the concentration of S2O32- increases, the color gradually fades. When the concentration is increased to 0.001 mol/L, the blue color completely fades, and the solution becomes colorless, as shown in Figure 6. When the content of thiosulfate in the solution is too high, this method can be used to directly judge by observing the color change.
FIGURE 6: Image of decreasing blue color vs. sodium thiosulfate concentration (from left to right) 0.000 mol/L to 0.001 mol/L, respectively.
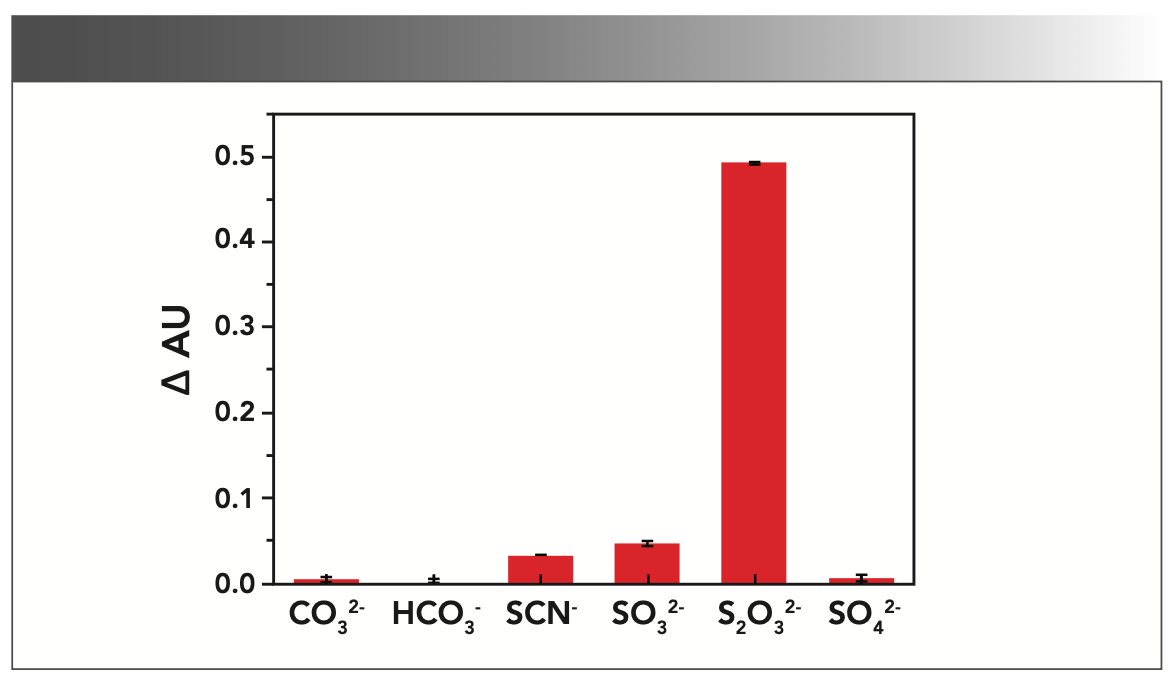
Effects of Interfering Substances
The acid ions such as CO32-, HCO3-, SCN-, SO32-, and SO42- coexisting in the desulfurization solution may interfere with the determination of the concentration of S2O32- acid ions. Therefore, the selectivity of this method was determined, in the same reaction system. We added 0.2 mL of 0.01 mol/L S2O32- and 2 mL of 0.01 mol/L coexisting interference ions; the results are shown in Figure 7, which show that the possibility of interference of these coexisting ions is very small, the pretreatment process is not required, and the concentration of S2O32- in the desulfurization solution can be directly measured.
FIGURE 7: Effect of interfering substances on absorbance (AU).
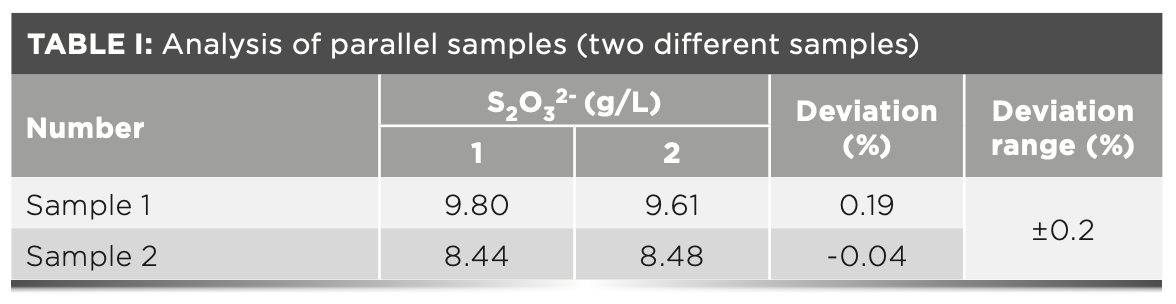
Determination of Samples
The desulfurization solutions samples of SD Steel Rizhao Co., Ltd. Coking Plant were analyzed in practice. The collected samples were preliminarily filtered and tested in parallel in accordance with the experimental protocol (because the subsequent experiments need to be compared with the test results of the coking laboratory, the measurement unit is unified to g/L). The results show that the analysis deviation is small, within the tolerance of ± 0.2%, as shown in Table I.
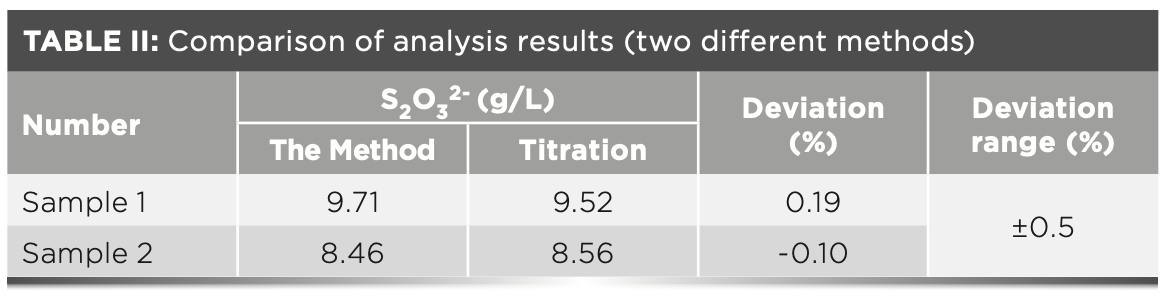
At the same time, to verify the reliability of the method, a comparison analysis was performed with the test results provided by the coking labo- ratory on the same day; the results show that the analysis deviation is small, within the allowable error of ± 0.5%, as shown in Table II. It is further verified that the method can detect thiosulfate ions in the desulfurization solution, and the measurement results are accurate and reliable.
Conclusions
We have developed a new method for the spectrophotometric determination of thiosulfate using methylene blue as an oxidant. Methylene blue will have different colors in the oxidized and reduced states. When reacting with thiosulfate, its absorbance value will gradually decrease with the disappearance of color. It was found that, even if a small amount of thiosulfate was added, the absorbance value of methylene blue changed. Based on this phenomenon, we designed a simple method to selectively determine the content of thiosulfate in the desulfurization solution with satisfactory results. At the same time, it has great environmental protection significance.
References
(1) Chen, K. Y.; Morris, J. C. Kinetics of Oxidation of Aqueous Sulfide by Oxygen. Environ. Sci. Tech. 1972, 6 (6),529–537. DOI: 10.1021/es60065a008
(2) Steudel, R. Mechanism for the Formation of Elemental Sulfur from Aqueous Sulfide in Chemical and Microbiological Desulfurization Processes Ind. Eng. Chem. Res. 1996, 35 (4), 1417–1423. DOI: 10.1021/ie950558t
(3) Fischer, H.; Schulz-Ekloff, G.; Woehrle, D. Oxidation of Aqueous Sulfide Solutions by Dioxygen Part I: Autoxidation Reaction. Chem. Eng. Technol. 1997, 20 (7), 462–468. DOI: 10.1002/ceat.270200705
(4) Luo, G. S.; Pan, S.; Liu, J. G. Use of the Electrodialysis Process to Concentrate a Formic Acid Solution. Desalination 2002, 150 (3), 227–234. DOI: 10.1016/S0011-9164(02)00978-5
(5) Liu, R.; Frost, R. L.; Martens, W. N. Absorption of the Selenite Anion from Aqueous Solutions by Thermally Activated Layered Double Hydroxide. Water Research 2009, 43 (5), 1323–1329. DOI: 10.1016/j.watres.2008.12.030
(6) Mani, K. N. Electrodialysis Water Splitting Technology. J. Membr. Sci. 1991, 58 (2), 117–138. DOI: 10.1016/S0376-7388(00)82450-3
(7) Kamyshny, A.; Goifman, A.; Gun, J.; Dan. R.; Lev, O. Equilibrium Distribution of Polysulfide Ions in Aqueous Solutions at 25 °C: A New Approach for the Study of Polysulfides' Equilibria. Environ. Sci. Technol. 2004, 38 (24), 6633-6644. DOI: 10.1021/es049514e
(8) Rickard, D.; Luther, G.W. Chemistry of Iron Sulfides. Chem. Rev. 2007, 107 (2), 514–562. DOI: 10.1021/cr0503658
(9) Kleinjan, W. E.; Keizer, A. D.; Janssen, A. J. H. Equilibrium of the Reaction Between Dissolved Sodium Sulfide and Biologically Produced Sulfur. Colloids Surf., B Biointerfaces 2005, 43 (3–4), 228–237. DOI: 10.1016/j.colsurfb.2005.05.004
(10) Shu, H. Y. Determination of Sodium Thiosulphate in Desulfurization Liquid. Jiangxi Metallurgy 2002, 3, 38–39.
(11) Rui-Jin, M. A.; Zheng-Xiang, Y. U.; Wang, X. Y.; Ding, W.; Yan, D. U. Determination Method of Sodium Thiosulfate and Sodium Sulfite in PDS Desulfurization Liquid. Chemical Technology and Development 2012, 11, 56–57.
(12) Lian, S.; Chi, H. A. T.; Kang, Z.; Yang, L.; Wong, N.; Lee, S. T. Photo-Controlled Redox of Hydrogen-Terminated Silicon Nanowire Established by the Reversible Color Alteration of Methylene Blue. Mater. Res. Bull. 2012, 47 (5), 1119–1122. DOI: 10.1016/j.materresbull.2012.02.016
(13) Pensini, E.; Elsayed, A.; Macias Rodriguez, B.; Marangoni, A. G.; Singh, A.; Sleep, B.; Hayward, G.; Lamont, K.; Collier, C. M. In Situ Trapping and Treating of Hexavalent Chromium Using Scleroglucan-Based Fluids: A Proof of Concept. Colloids Surf., A 2018, 559, 192–200 (2018). DOI: 10.1016/j.colsurfa.2018.09.051
Xiuxiu Zhao, Ming Xu, Chunying Ma, Yingying Ma, and Xianji Ma are with SD Steel Rizhao Co.,Ltd, in Rizhao, China. Direct correspondence to: zx860164430@163.com ●

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.